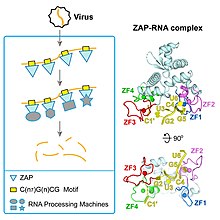
Interferons are a group of signaling proteins made and released by host cells in response to the presence of several viruses. In a typical scenario, a virus-infected cell will release interferons causing nearby cells to heighten their anti-viral defenses.

Antiviral drugs are a class of medication used for treating viral infections. Most antivirals target specific viruses, while a broad-spectrum antiviral is effective against a wide range of viruses. Unlike most antibiotics, antiviral drugs do not destroy their target pathogen; instead they inhibit its development.

Defective interfering particles (DIPs), also known as defective interfering viruses, are spontaneously generated virus mutants in which a critical portion of the particle's genome has been lost due to defective replication or non-homologous recombination. The mechanism of their formation is presumed to be as a result of template-switching during replication of the viral genome, although non-replicative mechanisms involving direct ligation of genomic RNA fragments have also been proposed. DIPs are derived from and associated with their parent virus, and particles are classed as DIPs if they are rendered non-infectious due to at least one essential gene of the virus being lost or severely damaged as a result of the defection. A DIP can usually still penetrate host cells, but requires another fully functional virus particle to co-infect a cell with it, in order to provide the lost factors.

The hepatitis C virus (HCV) is a small, enveloped, positive-sense single-stranded RNA virus of the family Flaviviridae. The hepatitis C virus is the cause of hepatitis C and some cancers such as liver cancer and lymphomas in humans.
Viral pathogenesis is the study of the process and mechanisms by which viruses cause diseases in their target hosts, often at the cellular or molecular level. It is a specialized field of study in virology.

Murine respirovirus, formerly Sendai virus (SeV) and previously also known as murine parainfluenza virus type 1 or hemagglutinating virus of Japan (HVJ), is an enveloped,150-200 nm in diameter, a negative sense, single-stranded RNA virus of the family Paramyxoviridae. It typically infects rodents and it is not pathogenic for humans or domestic animals. Sendai virus (SeV) is a member of genus Respirovirus. The virus was isolated in the city of Sendai in Japan in the early 1950s. Since then, it has been actively used in research as a model pathogen. The virus is infectious for many cancer cell lines, has oncolytic properties demonstrated in animal models and in naturally-occurring cancers in animals. SeV's ability to fuse eukaryotic cells and to form syncytium was used to produce hybridoma cells capable of manufacturing monoclonal antibodies in large quantities. Recent applications of SeV-based vectors include the reprogramming of somatic cells into induced pluripotent stem cells and vaccines creation. For vaccination purpose the Sendai virus-based constructs could be delivered in a form of nasal drops, which may be beneficial in inducing a mucosal immune response. SeV has several features that are important in a vector for a successful vaccine: the virus does not integrate into the host genome, it does not undergo genetic recombination, it replicates only in the cytoplasm without DNA intermediates or a nuclear phase and it is not causing any disease in humans or domestic animals. Sendai virus is used as a backbone for vaccine development against Mycobacterium tuberculosis that causes tuberculosis, against HIV-1 that causes AIDS and against other viruses, including those that cause severe respiratory infections in children. The latter include Human Respiratory Syncytial Virus (HRSV), Human Metapneumovirus (HMPV) and Human Parainfluenza Viruses (HPIV). The vaccine studies against Mycobacterium tuberculosis, HMPV, HPIV1 and, HPIV2 are in pre-clinical stage, against HRSV phase I clinical trail has been completed. The phase I clinical studies of SeV-based vaccination were also completed for HPIV1. They were done in adults and in 3- to 6-year-old children. As a result of vaccination against HPIV1 the significant boost in virus-specific neutralizing antibodies was observed. The SeV-based vaccine development against HIV-1 have reached phase II clinical trial. Fudan University in collaboration with ID Pharma Co. Ltd. is engaged in development of the vaccine for COVID-19 prevention. SeV serves as a vaccine backbone vector in the project.
NSP1 (NS53), the product of rotavirus gene 5, is a nonstructural RNA-binding protein that contains a cysteine-rich region and is a component of early replication intermediates. RNA-folding predictions suggest that this region of the NSP1 mRNA can interact with itself, producing a stem-loop structure similar to that found near the 5'-terminus of the NSP1 mRNA.

RIG-I is a cytosolic pattern recognition receptor (PRR) responsible for the type-1 interferon (IFN1) response. RIG-I is an essential molecule in the innate immune system for recognizing cells that have been infected with a virus. These viruses can include West Nile virus, Japanese Encephalitis virus, influenza A, Sendai virus, flavivirus, and coronaviruses. RIG-I is structurally considered a helical ATP-dependent DExD/H box RNA helicase, that recognizes short viral double-stranded RNA (dsRNA) in the cytosol during a viral infection or other irregular RNAs. Once activated by the dsRNA, the N-terminus caspase activation and recruitment domains (CARDs) migrate and bind with CARDs attached to mitochondrial antiviral signaling protein (MAVS) to activate the signaling pathway for IFN1. IFN1s have three main functions: to limit the virus from spreading to nearby cells, promote an innate immune response, including inflammatory responses, and help activate the adaptive immune system. Other studies have shown that in different microenvironments, such as in cancerous cells, RIG-I has more functions other than viral recognition. RIG-I orthologs are found in mammals, geese, ducks, some fish, and some reptiles. RIG-I is in most cells, including various innate immune system cells, and is usually in an inactive state. Knockout mice that have been designed to have a deleted or non-functioning RIG-I gene are not healthy and typically die embryonically. If they survive, the mice have serious developmental dysfunction. The stimulator of interferon genes STING antagonizes RIG-1 by binding its N-terminus, probably as to avoid overactivation of RIG-1 signaling and the associated autoimmunity.

Interferon-induced GTP-binding protein Mx1 is a protein that in humans is encoded by the MX1 gene.

Interferon alpha-2 is a protein that in humans is encoded by the IFNA2 gene.

MDA5 is a RIG-I-like receptor dsRNA helicase enzyme that is encoded by the IFIH1 gene in humans. MDA5 is part of the RIG-I-like receptor (RLR) family, which also includes RIG-I and LGP2, and functions as a pattern recognition receptor capable of detecting viruses. It is generally believed that MDA5 recognizes double stranded RNA (dsRNA) over 2000nts in length, however it has been shown that whilst MDA5 can detect and bind to cytoplasmic dsRNA, it is also activated by a high molecular weight RNA complex composed of ssRNA and dsRNA. For many viruses, effective MDA5-mediated antiviral responses are dependent on functionally active LGP2. The signaling cascades in MDA5 is initiated via CARD domain. Some observations made in cancer cells show that MDA5 also interacts with cellular RNA is able to induce an autoinflammatory response.

Interferon-induced transmembrane protein 1 is a protein that in humans is encoded by the IFITM1 gene. IFITM1 has also recently been designated CD225. This protein has several additional names: fragilis, IFI17 [interferon-induced protein 17], 9-27 [Interferon-inducible protein 9-27] and Leu13.
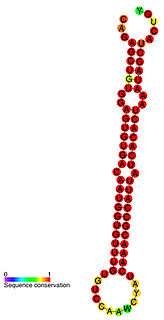
miR-122 is a miRNA that is conserved among vertebrate species. miR-122 is not present in invertebrates, and no close paralogs of miR-122 have been detected. miR-122 is highly expressed in the liver, where it has been implicated as a regulator of fatty-acid metabolism in mouse studies. Reduced miR-122 levels are associated with hepatocellular carcinoma. miR-122 also plays an important positive role in the regulation of hepatitis C virus replication.
Adolfo García-Sastre,(born in Burgos, 10 October 1964) is a Spanish professor of Medicine and Microbiology and co-director of the Global Health & Emerging Pathogens Institute at the Icahn School of Medicine at Mount Sinai in New York City. His research into the biology of influenza viruses has been at the forefront of medical advances in epidemiology.
RIG-like receptors are a type of intracellular pattern recognition receptor involved in the recognition of viruses by the innate immune system. RIG-I is the best characterized receptor within the RIG-I like receptor (RLR) family. Together with MDA5 and LGP2, this family of cytoplasmic pattern recognition receptors (PRRs) are sentinels for intracellular viral RNA that is a product of viral infection. The RLR receptors provide frontline defence against viral infections in most tissues.

Radical S-adenosyl methionine domain-containing protein 2 is a protein that in humans is encoded by the RSAD2 gene. RSAD2 is a multifunctional protein in viral processes that is an interferon stimulated gene. It has been reported that viperin could be induced by either IFN-dependent or IFN-independent pathways and certain viruses may use viperin to increase their infectivity.
In molecular biology, the protein family Dispanin is another name for Interferon-induced transmembrane protein (IFITM). This refers to a family of protein domains which have a specific formation, or in other words, topology containing two alpha helices in within the cell membrane which are called two transmembrane proteins. This includes proteins such as CD225. The function of this protein family is to inhibit cell invasion of many harmful, pathogenic viruses, such as HIV. Henceforth, they are being intensively studied in the hope of drug discovery. They mediate the immune response by interferons.
An interferon-stimulated gene (ISG) is a gene that can be expressed in response to stimulation by interferon. Interferons bind to receptors on the surface of a cell, initiating protein signaling pathways within the cell. This interaction leads to the expression of a subset of genes involved in the innate immune system response. ISGs are commonly expressed in response to viral infection, but also during bacterial infection and in the presence of parasites.

Interferon-induced transmembrane protein 2 is a protein that in humans is encoded by the IFITM2 gene. IFITM1 is a member of the IFITM family which is encoded by IFITM genes.
HSV epigenetics is the epigenetic modification of herpes simplex virus (HSV) genetic code.