In physical chemistry, supersaturation occurs with a solution when the concentration of a solute exceeds the concentration specified by the value of solubility at equilibrium. Most commonly the term is applied to a solution of a solid in a liquid, but it can also be applied to liquids and gases dissolved in a liquid. A supersaturated solution is in a metastable state; it may return to equilibrium by separation of the excess of solute from the solution, by dilution of the solution by adding solvent, or by increasing the solubility of the solute in the solvent.

Oxygen cycle refers to the movement of oxygen through the atmosphere (air), biosphere (plants and animals) and the lithosphere (the Earth’s crust). The oxygen cycle demonstrates how free oxygen is made available in each of these regions, as well as how it is used. The oxygen cycle is the biogeochemical cycle of oxygen atoms between different oxidation states in ions, oxides, and molecules through redox reactions within and between the spheres/reservoirs of the planet Earth. The word oxygen in the literature typically refers to the most common oxygen allotrope, elemental/diatomic oxygen (O2), as it is a common product or reactant of many biogeochemical redox reactions within the cycle. Processes within the oxygen cycle are considered to be biological or geological and are evaluated as either a source (O2 production) or sink (O2 consumption).
The oxygen minimum zone (OMZ), sometimes referred to as the shadow zone, is the zone in which oxygen saturation in seawater in the ocean is at its lowest. This zone occurs at depths of about 200 to 1,500 m (700–4,900 ft), depending on local circumstances. OMZs are found worldwide, typically along the western coast of continents, in areas where an interplay of physical and biological processes concurrently lower the oxygen concentration and restrict the water from mixing with surrounding waters, creating a "pool" of water where oxygen concentrations fall from the normal range of 4–6 mg/L to below 2 mg/L.

Oxygen saturation is a relative measure of the concentration of oxygen that is dissolved or carried in a given medium as a proportion of the maximal concentration that can be dissolved in that medium at the given temperature. It can be measured with a dissolved oxygen probe such as an oxygen sensor or an optode in liquid media, usually water. The standard unit of oxygen saturation is percent (%).
The pedosphere is the outermost layer of the Earth that is composed of soil and subject to soil formation processes. It exists at the interface of the lithosphere, atmosphere, hydrosphere and biosphere. The pedosphere is the skin of the Earth and only develops when there is a dynamic interaction between the atmosphere, biosphere, lithosphere and the hydrosphere. The pedosphere is the foundation of terrestrial life on Earth.

In oceanic biogeochemistry, the solubility pump is a physico-chemical process that transports carbon as dissolved inorganic carbon (DIC) from the ocean's surface to its interior.
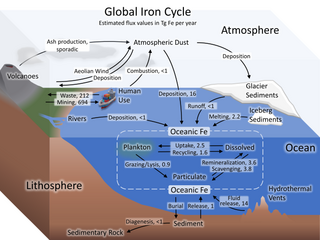
The iron cycle (Fe) is the biogeochemical cycle of iron through the atmosphere, hydrosphere, biosphere and lithosphere. While Fe is highly abundant in the Earth's crust, it is less common in oxygenated surface waters. Iron is a key micronutrient in primary productivity, and a limiting nutrient in the Southern ocean, eastern equatorial Pacific, and the subarctic Pacific referred to as High-Nutrient, Low-Chlorophyll (HNLC) regions of the ocean.

Biogenic silica (bSi), also referred to as opal, biogenic opal, or amorphous opaline silica, forms one of the most widespread biogenic minerals. For example, microscopic particles of silica called phytoliths can be found in grasses and other plants.
Ocean stratification is the natural separation of an ocean's water into horizontal layers by density, which is generally stable because warm water floats on top of cold water, and heating is mostly from the sun, which reinforces that arrangement. Stratification is reduced by wind-forced mechanical mixing, but reinforced by convection. Stratification occurs in all ocean basins and also in other water bodies. Stratified layers are a barrier to the mixing of water, which impacts the exchange of heat, carbon, oxygen and other nutrients. The surface mixed layer is the uppermost layer in the ocean and is well mixed by mechanical (wind) and thermal (convection) effects. Climate change is causing the upper ocean stratification to increase.

Siliceous ooze is a type of biogenic pelagic sediment located on the deep ocean floor. Siliceous oozes are the least common of the deep sea sediments, and make up approximately 15% of the ocean floor. Oozes are defined as sediments which contain at least 30% skeletal remains of pelagic microorganisms. Siliceous oozes are largely composed of the silica based skeletons of microscopic marine organisms such as diatoms and radiolarians. Other components of siliceous oozes near continental margins may include terrestrially derived silica particles and sponge spicules. Siliceous oozes are composed of skeletons made from opal silica SiO2·nH2O, as opposed to calcareous oozes, which are made from skeletons of calcium carbonate (CaCO3·nH2O) organisms (i.e. coccolithophores). Silica (Si) is a bioessential element and is efficiently recycled in the marine environment through the silica cycle. Distance from land masses, water depth and ocean fertility are all factors that affect the opal silica content in seawater and the presence of siliceous oozes.

Ocean deoxygenation is the reduction of the oxygen content in different parts of the ocean due to human activities. It occurs firstly in coastal zones where eutrophication has driven some quite rapid declines in oxygen to very low levels. This type of ocean deoxygenation is also called "dead zones". Secondly, there is now an ongoing reduction in oxygen levels in the open ocean: naturally occurring low oxygen areas are now expanding slowly. This expansion is happening as a consequence of human caused climate change. The resulting decrease in oxygen content of the oceans poses a threat to marine life, as well as to people who depend on marine life for nutrition or livelihood. Ocean deoxygenation poses implications for ocean productivity, nutrient cycling, carbon cycling, and marine habitats.

Environmental oxygenation can be important to the sustainability of a particular ecosystem. Insufficient oxygen may occur in bodies of water such as ponds and rivers, tending to suppress the presence of aerobic organisms such as fish. Deoxygenation increases the relative population of anaerobic organisms such as plants and some bacteria, resulting in fish kills and other adverse events. The net effect is to alter the balance of nature by increasing the concentration of anaerobic over aerobic species.
Marine chemistry, also known as ocean chemistry or chemical oceanography, is influenced by plate tectonics and seafloor spreading, turbidity currents, sediments, pH levels, atmospheric constituents, metamorphic activity, and ecology. The field of chemical oceanography studies the chemistry of marine environments including the influences of different variables. Marine life has adapted to the chemistries unique to earth's oceans, and marine ecosystems are sensitive to changes in ocean chemistry.
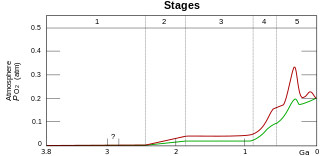
Before photosynthesis evolved, Earth's atmosphere had no free oxygen (O2). Small quantities of oxygen were released by geological and biological processes, but did not build up in the atmosphere due to reactions with reducing minerals.

Hypoxia refers to low oxygen conditions. Normally, 20.9% of the gas in the atmosphere is oxygen. The partial pressure of oxygen in the atmosphere is 20.9% of the total barometric pressure. In water, oxygen levels are much lower, approximately 7 ppm or 0.0007% in good quality water, and fluctuate locally depending on the presence of photosynthetic organisms and relative distance to the surface.

The oceanic carbon cycle is composed of processes that exchange carbon between various pools within the ocean as well as between the atmosphere, Earth interior, and the seafloor. The carbon cycle is a result of many interacting forces across multiple time and space scales that circulates carbon around the planet, ensuring that carbon is available globally. The Oceanic carbon cycle is a central process to the global carbon cycle and contains both inorganic carbon and organic carbon. Part of the marine carbon cycle transforms carbon between non-living and living matter.

Shell growth in estuaries is an aspect of marine biology that has attracted a number of scientific research studies. Many groups of marine organisms produce calcified exoskeletons, commonly known as shells, hard calcium carbonate structures which the organisms rely on for various specialized structural and defensive purposes. The rate at which these shells form is greatly influenced by physical and chemical characteristics of the water in which these organisms live. Estuaries are dynamic habitats which expose their inhabitants to a wide array of rapidly changing physical conditions, exaggerating the differences in physical and chemical properties of the water.
Evolution of metal ions in biological systems refers to the incorporation of metallic ions into living organisms and how it has changed over time. Metal ions have been associated with biological systems for billions of years, but only in the last century have scientists began to truly appreciate the scale of their influence. Major and minor metal ions have become aligned with living organisms through the interplay of biogeochemical weathering and metabolic pathways involving the products of that weathering. The associated complexes have evolved over time.

The Arctic ocean covers an area of 14,056,000 square kilometers, and supports a diverse and important socioeconomic food web of organisms, despite its average water temperature being 32 degrees Fahrenheit. Over the last three decades, the Arctic Ocean has experienced drastic changes due to climate change. One of the changes is in the acidity levels of the ocean, which have been consistently increasing at twice the rate of the Pacific and Atlantic oceans. Arctic Ocean acidification is a result of feedback from climate system mechanisms, and is having negative impacts on Arctic Ocean ecosystems and the organisms that live within them.

The manganese cycle is the biogeochemical cycle of manganese through the atmosphere, hydrosphere, biosphere and lithosphere. There are bacteria that oxidise manganese to insoluble oxides, and others that reduce it to Mn2+ in order to use it.

















