
Integrins are transmembrane receptors that help cell-cell and cell-extracellular matrix (ECM) adhesion. Upon ligand binding, integrins activate signal transduction pathways that mediate cellular signals such as regulation of the cell cycle, organization of the intracellular cytoskeleton, and movement of new receptors to the cell membrane. The presence of integrins allows rapid and flexible responses to events at the cell surface.

Fibronectin is a high-molecular weight glycoprotein of the extracellular matrix that binds to membrane-spanning receptor proteins called integrins. Fibronectin also binds to other extracellular matrix proteins such as collagen, fibrin, and heparan sulfate proteoglycans.

In biology, the extracellular matrix (ECM), also called intercellular matrix (ICM), is a network consisting of extracellular macromolecules and minerals, such as collagen, enzymes, glycoproteins and hydroxyapatite that provide structural and biochemical support to surrounding cells. Because multicellularity evolved independently in different multicellular lineages, the composition of ECM varies between multicellular structures; however, cell adhesion, cell-to-cell communication and differentiation are common functions of the ECM.
Cell adhesion molecules (CAMs) are a subset of cell surface proteins that are involved in the binding of cells with other cells or with the extracellular matrix (ECM), in a process called cell adhesion. In essence, CAMs help cells stick to each other and to their surroundings. CAMs are crucial components in maintaining tissue structure and function. In fully developed animals, these molecules play an integral role in generating force and movement and consequently ensuring that organs are able to execute their functions normally. In addition to serving as "molecular glue", CAMs play important roles in the cellular mechanisms of growth, contact inhibition, and apoptosis. Aberrant expression of CAMs may result in a wide range of pathologies, ranging from frostbite to cancer.
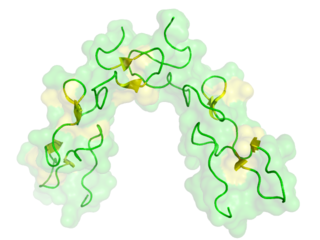
Disintegrins are a family of small proteins from viper venoms that function as potent inhibitors of both platelet aggregation and integrin-dependent cell adhesion.

Versican is a large extracellular matrix proteoglycan that is present in a variety of human tissues. It is encoded by the VCAN gene.
Targeted drug delivery, sometimes called smart drug delivery, is a method of delivering medication to a patient in a manner that increases the concentration of the medication in some parts of the body relative to others. This means of delivery is largely founded on nanomedicine, which plans to employ nanoparticle-mediated drug delivery in order to combat the downfalls of conventional drug delivery. These nanoparticles would be loaded with drugs and targeted to specific parts of the body where there is solely diseased tissue, thereby avoiding interaction with healthy tissue. The goal of a targeted drug delivery system is to prolong, localize, target and have a protected drug interaction with the diseased tissue. The conventional drug delivery system is the absorption of the drug across a biological membrane, whereas the targeted release system releases the drug in a dosage form. The advantages to the targeted release system is the reduction in the frequency of the dosages taken by the patient, having a more uniform effect of the drug, reduction of drug side-effects, and reduced fluctuation in circulating drug levels. The disadvantage of the system is high cost, which makes productivity more difficult, and the reduced ability to adjust the dosages.

Endoglin (ENG) is a type I membrane glycoprotein located on cell surfaces and is part of the TGF beta receptor complex. It is also commonly referred to as CD105, END, FLJ41744, HHT1, ORW and ORW1. It has a crucial role in angiogenesis, therefore, making it an important protein for tumor growth, survival and metastasis of cancer cells to other locations in the body.

Thrombospondin 1, abbreviated as THBS1, is a protein that in humans is encoded by the THBS1 gene.

Sanford Burnham Prebys is a 501(c)(3) non-profit medical research institute focusing on basic and translational research, with major research programs in cancer, neurodegeneration, diabetes, infectious, inflammatory, and childhood diseases. The institute also specializes in stem cell research and drug discovery technologies.

In molecular biology, CD18 is an integrin beta chain protein that is encoded by the ITGB2 gene in humans. Upon binding with one of a number of alpha chains, CD18 is capable of forming multiple heterodimers, which play significant roles in cellular adhesion and cell surface signaling, as well as important roles in immune responses. CD18 also exists in soluble, ligand binding forms. Deficiencies in CD18 expression can lead to adhesion defects in circulating white blood cells in humans, reducing the immune system's ability to fight off foreign invaders.

Tenascin C (TN-C) is a glycoprotein that in humans is encoded by the TNC gene. It is expressed in the extracellular matrix of various tissues during development, disease or injury, and in restricted neurogenic areas of the central nervous system. Tenascin-C is the founding member of the tenascin protein family. In the embryo it is made by migrating cells like the neural crest; it is also abundant in developing tendons, bone and cartilage.

Integrin beta-6 is a protein that in humans is encoded by the ITGB6 gene. It is the β6 subunit of the integrin αvβ6. Integrins are αβ heterodimeric glycoproteins which span the cell’s membrane, integrating the outside and inside of the cell. Integrins bind to specific extracellular proteins in the extracellular matrix or on other cells and subsequently transduce signals intracellularly to affect cell behaviour. One α and one β subunit associate non-covalently to form 24 unique integrins found in mammals. While some β integrin subunits partner with multiple α subunits, β6 associates exclusively with the αv subunit. Thus, the function of ITGB6 is entirely associated with the integrin αvβ6.

Integrin alpha-9 is a protein that in humans is encoded by the ITGA9 gene. Cytogenetic location: 3p22.2
Erkki Ruoslahti is a cancer researcher and distinguished professor at Sanford Burnham Prebys Medical Discovery Institute. He moved from Finland to the United States in 1976.
Lee Byung-heon (Korean: 이병헌) is a professor of biochemistry and cell biology in the school of medicine at Kyungpook National University (KNU), South Korea. He received his M.D. license from Korean Medical Association in 1989. He received his B.S. from the school of medicine, KNU, in 1989, and his M.S. and Ph.D. in biochemistry from KNU in 1991 and 1995. He was an assistant professor in the school of medicine at Dongguk University in 1996–2001 and a visiting investigator in the Sanford-Burnham Medical Research Institute, La Jolla, United States, in 2001–2003. He joined KNU in 2003. He is currently a member of the Korean Society for Biochemistry and Molecular Biology, the American Association for Cancer Research, and the American Society of Molecular Imaging. His main research interest is “discovery of tissue-specific homing peptides using phage display and their applications to molecular imaging and targeted therapy”. He is currently carrying out projects for the identification of homing peptides to tumor and atherosclerotic plaque and of phosphatidylserine- and blood clotting factor XIIIa-specific peptide ligands. He has published over 30 peer-reviewed papers, book chapters, and review articles. He has also filed several patents.
Invasins are a class of bacterial proteins associated with the penetration of pathogens into host cells. Invasins play a role in promoting entry during the initial stage of infection.

Integrin-like receptors (ILRs) are found in plants and carry unique functional properties similar to true integrin proteins. True homologs of integrins exist in mammals, invertebrates, and some fungi but not in plant cells. Mammalian integrins are heterodimer transmembrane proteins that play a large role in bidirectional signal transduction. As transmembrane proteins, integrins connect the extracellular matrix (ECM) to the plasma membrane of the animal cell. The extracellular matrix of plant cells, fungi, and some protist is referred to as the cell wall. The plant cell wall is composed of a tough cellulose polysaccharide rather than the collagen fibers of the animal ECM. Even with these differences, research indicates that similar proteins involved in the interaction between the ECM and animals cells are also involved in the interaction of the cell wall and plant cells.
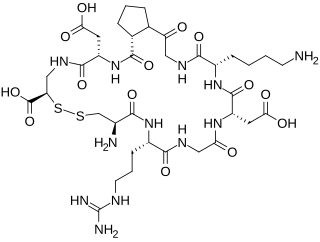
iRGD is a 9-amino acid cyclic peptide and a molecular mimicry agent that was originally identified in an in vivo screening of phage display libraries in tumor-bearing mice. The peptide was able to home to tumor tissues, but in contrast to standard RGD peptides, also spread much more extensively into extravascular tumor tissue. It was later identified that this extravasation and transport through extravascular tumor tissue was due to the bifunctional action of the molecule: after the initial RGD-mediated tumor homing, another pharmacological motif is able to manipulate tumor microenvironment, making it temporarily accessible to circulating drugs. This second step is mediated through specific secondary binding to neuropilin-1 receptor, and subsequent activation of a trans-tissue pathway, dubbed the C-end Rule, or CendR pathway.
CendR is a position-dependent protein motif that regulates cellular uptake and vascular permeability through interaction with neuropilin-1. The CendR motif has a consensus (R/K)XX(R/K) and it is able to interact with its receptor only when the second basic residue is exposed at the C-terminus.






















