Related Research Articles
Ferrocene is an organometallic compound with the formula Fe(C5H5)2. The molecule is a complex consisting of two cyclopentadienyl rings bound to a central iron atom. It is an orange solid with a camphor-like odor, that sublimes above room temperature, and is soluble in most organic solvents. It is remarkable for its stability: it is unaffected by air, water, strong bases, and can be heated to 400 °C without decomposition. In oxidizing conditions it can reversibly react with strong acids to form the ferrocenium cation Fe(C5H5)+2. Ferrocene and the ferrocenium cation are sometimes abbreviated as Fc and Fc+ respectively.

Scandium(III) chloride is the inorganic compound with the formula ScCl3. It is a white, high-melting ionic compound, which is deliquescent and highly water-soluble. This salt is mainly of interest in the research laboratory. Both the anhydrous form and hexahydrate (ScCl3•6H2O) are commercially available.

Cupferron is jargon for the ammonium salt of the conjugate base derived from N-nitroso-N-phenylhydroxylamine. It once was a common reagent for the complexation of metal ions, being of interest in the area of qualitative inorganic analysis. Its formula is NH4[C6H5N(O)NO]. The anion binds to metal cations through the two oxygen atoms, forming five-membered chelate rings.

Tritellurium dichloride is the inorganic compound with the formula Te3Cl2. It is one of the more stable lower chlorides of tellurium.
Titanium(III) phosphide (TiP) is an inorganic chemical compound of titanium and phosphorus. Normally encountered as a grey powder, it is a metallic conductor with a high melting point. It is not attacked by common acids or water. Its physical properties stand in contrast to the group 1 and group 2 phosphides that contain the P3− anion (such as Na3P), which are not metallic and are readily hydrolysed. Titanium phosphide is classified as a "metal-rich phosphide", where extra valence electrons from the metal are delocalised.

Helimagnetism is a form of magnetic ordering where spins of neighbouring magnetic moments arrange themselves in a spiral or helical pattern, with a characteristic turn angle of somewhere between 0 and 180 degrees. It results from the competition between ferromagnetic and antiferromagnetic exchange interactions. It is possible to view ferromagnetism and antiferromagnetism as helimagnetic structures with characteristic turn angles of 0 and 180 degrees respectively. Helimagnetic order breaks spatial inversion symmetry, as it can be either left-handed or right-handed in nature.
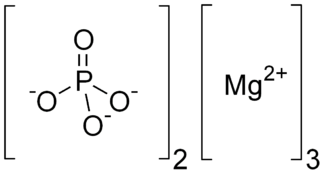
Trimagnesium phosphate describes inorganic compounds with formula Mg3(PO4)2.xH2O. They are magnesium acid salts of phosphoric acid, with varying amounts of water of crystallization: x = 0, 5, 8, 22.
Silver selenite is an inorganic compound of formula Ag2SeO3.
Iron phosphide is a chemical compound of iron and phosphorus, with a formula of FeP. Its physical appearance is grey, hexagonal needles.

Osmium dioxide is an inorganic compound with the formula OsO2. It exists as brown to black crystalline powder, but single crystals are golden and exhibit metallic conductivity. The compound crystallizes in the rutile structural motif, i.e. the connectivity is very similar to that in the mineral rutile.

Diethylaluminium chloride, abbreviated DEAC, is an organoaluminium compound. Although usually given the chemical formula (C2H5)2AlCl, it exists as a dimer, [(C2H5)2AlCl]2 It is a precursor to Ziegler-Natta catalysts employed for the production of polyolefins. The compound is also a Lewis acid, useful in organic synthesis. The compound is a colorless waxy solid, but is usually handled as a solution in hydrocarbon solvents. It is highly reactive, even pyrophoric.
Tellurium iodide is an inorganic compound with the formula TeI. Two forms are known. Their structures differ from the other monohalides of tellurium. There are three subiodides of tellurium, α-TeI, β-TeI, and Te2I, and one tellurium tetraiodide.
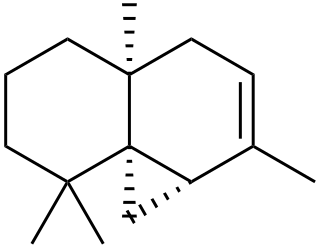
Thujopsene is a natural chemical compound, classified as a sesquiterpene, with the molecular formula C15H24.
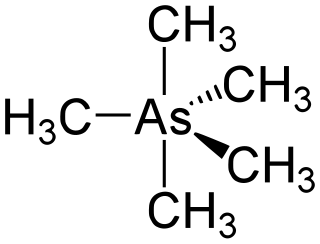
Pentamethylarsenic (or pentamethylarsorane)is an organometalllic compound containing five methyl groups bound to an arsenic atom with formula As(CH3)5. It is an example of a hypervalent compound. The molecular shape is trigonal bipyramid.

Pentamethylantimony or pentamethylstiborane is an organometalllic compound containing five methyl groups bound to an antimony atom with formula Sb(CH3)5. It is an example of a hypervalent compound. The molecular shape is trigonal bipyramid. Some other antimony(V) organometallic compounds include pentapropynylantimony (Sb(CCCH3)5) and pentaphenyl antimony (Sb(C6H5)5). Other known pentamethyl-pnictides include pentamethylbismuth and pentamethylarsenic.

Trithiapentalene is an organic bicyclic molecule containing two sulfur heterocycles. Its 10-π aromatic structure is similar to naphthalene. There has been a literature dispute about whether the connectivity among the three sulfur atoms is a case of rapid tautomerization between two valence tautomers or a 3-center 4-electron bond.

Karin Aurivillius (1920–1982) was a Swedish chemist and crystallographer at the University of Lund, Sweden. She determined the crystal structures of many mercury compounds.
Antimony(III) oxide hydroxide nitrate is an inorganic compound with the chemical formula Sb4O4(OH)2(NO3)2. It is one of the very few nitrates of antimony. No evidence for a simple trinitrate has been reported. According to X-ray crystallography, its structure consists of cationic layers of antimony oxide/hydroxide with intercalated nitrate anions. This compound is produced by the reaction of antimony(III) oxide and nitric acid and 110 °C.

Praseodymium(III) iodide is an inorganic salt, consisting of the rare-earth metal praseodymium and iodine, with the chemical formula PrI3. It forms green crystals. It is soluble in water.
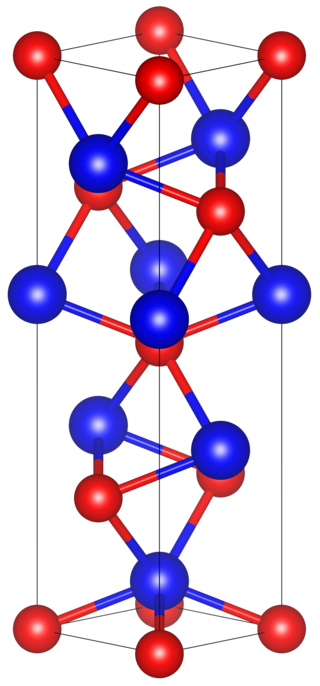
Tantalum arsenide is a compound of tantalum and arsenic with the formula TaAs. It is notable as being the first topological Weyl semimetal that was identified and characterized by ARPES.
References
- ↑ "Arne Haaland". Store norske leksikon (in Norwegian). Retrieved 12 September 2012.
- ↑ "Norske medlemmer" (in Norwegian). Norwegian Academy of Science and Letters . Retrieved 27 December 2021.
- ↑ Almenningen, A.; Bastiansen, O.; Haaland, A. (June 1964). "Molecular Structure of Dicyclopentadienylberyllium (C5H5)2Be". The Journal of Chemical Physics. 40 (11): 3434–3437. Bibcode:1964JChPh..40.3434A. doi:10.1063/1.1725019. ISSN 0021-9606.
- 1 2 Haaland, Arne (1968). "On the Molecular Structure of Di-cyclopentadienylberyllium (C5H5)2Be. II. Least Squares Refinement". Acta Chemica Scandinavica. 22: 3030–3032. doi: 10.3891/acta.chem.scand.22-3030 .
- ↑ Haaland, A.; Blomstrand, Rolf; Reunanen, Matti; Kyyhkynen, Hertta; Hoffman, Ragnar A.; Westerdahl, Anders (1965). "The Molecular Structure of Gaseous Dibenzene Chromium (C6H6)2Cr". Acta Chemica Scandinavica. 19: 41–46. doi: 10.3891/acta.chem.scand.19-0041 . ISSN 0904-213X.
- ↑ Almenningen, A.; Halvorsen, S.; Haaland, A.; Pihlaja, Kalevi; Schaumburg, Kjeld; Ehrenberg, L. (1971). "A Gas Phase Electron Diffraction Investigation of the Molecular Structures of Trimethylaluminium Monomer and Dimer". Acta Chemica Scandinavica. 25: 1937–1945. doi: 10.3891/acta.chem.scand.25-1937 . ISSN 0904-213X.
- ↑ Anderson, G. A. (1971). "MOLECULAR STRUCTURE OF TRIMETHYLALUMINIUM-TRIMETHYLAMINE BY ELECTRON DIFFRACTION". Journal of the Chemical Society D: Chemical Communications: 480.
- ↑ Denk, Michael; Lennon, Robert; Hayashi, Randy; West, Robert; Belyakov, Alexander V.; Verne, Hans P.; Haaland, Arne; Wagner, Matthias; Metzler, Nils (March 1994). "Synthesis and Structure of a Stable Silylene". Journal of the American Chemical Society. 116 (6): 2691–2692. doi:10.1021/ja00085a088. ISSN 0002-7863.
- ↑ Haaland, Arne. (2008). Molecules and models : the molecular structures of main group element compounds. Oxford: Oxford University Press. ISBN 978-0-19-923535-3. OCLC 173809048.
- ↑ Haaland, Arne (August 1989). "Covalent versus Dative Bonds to Main Group Metals, a Useful Distinction". Angewandte Chemie International Edition in English. 28 (8): 992–1007. doi:10.1002/anie.198909921. ISSN 0570-0833.
- ↑ Haaland, Arne (1979-11-01). "Molecular structure and bonding in the 3d metallocenes". Accounts of Chemical Research. 12 (11): 415–422. doi:10.1021/ar50143a006. ISSN 0001-4842.