
A myelodysplastic syndrome (MDS) is one of a group of cancers in which immature blood cells in the bone marrow do not mature, and as a result, do not develop into healthy blood cells. Early on, no symptoms typically are seen. Later, symptoms may include fatigue, shortness of breath, bleeding disorders, anemia, or frequent infections. Some types may develop into acute myeloid leukemia.
Aplastic anemia (AA) is a severe hematologic condition in which the body fails to make blood cells in sufficient numbers. Aplastic anemia is associated with cancer and various cancer syndromes. Blood cells are produced in the bone marrow by stem cells that reside there. Aplastic anemia causes a deficiency of all blood cell types: red blood cells, white blood cells, and platelets.
Neutropenia is an abnormally low concentration of neutrophils in the blood. Neutrophils make up the majority of circulating white blood cells and serve as the primary defense against infections by destroying bacteria, bacterial fragments and immunoglobulin-bound viruses in the blood. People with neutropenia are more susceptible to bacterial infections and, without prompt medical attention, the condition may become life-threatening.

Chronic lymphocytic leukemia (CLL) is a type of cancer in which the bone marrow makes too many lymphocytes. Early on, there are typically no symptoms. Later, non-painful lymph node swelling, feeling tired, fever, night sweats, or weight loss for no clear reason may occur. Enlargement of the spleen and low red blood cells (anemia) may also occur. It typically worsens gradually over years.
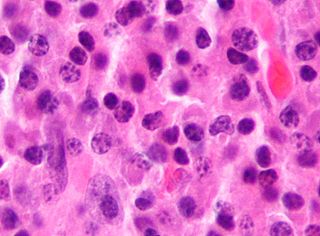
Tumors of the hematopoietic and lymphoid tissues or tumours of the haematopoietic and lymphoid tissues are tumors that affect the blood, bone marrow, lymph, and lymphatic system. Because these tissues are all intimately connected through both the circulatory system and the immune system, a disease affecting one will often affect the others as well, making aplasia, myeloproliferation and lymphoproliferation closely related and often overlapping problems. While uncommon in solid tumors, chromosomal translocations are a common cause of these diseases. This commonly leads to a different approach in diagnosis and treatment of hematological malignancies. Hematological malignancies are malignant neoplasms ("cancer"), and they are generally treated by specialists in hematology and/or oncology. In some centers "hematology/oncology" is a single subspecialty of internal medicine while in others they are considered separate divisions. Not all hematological disorders are malignant ("cancerous"); these other blood conditions may also be managed by a hematologist.
Evans syndrome is an autoimmune disease in which an individual's immune system attacks their own red blood cells and platelets, the syndrome can include immune neutropenia. These immune cytopenias may occur simultaneously or sequentially.

Fas ligand is a type-II transmembrane protein expressed on cytotoxic T lymphocytes and natural killer (NK) cells. Its binding with Fas receptor (FasR) induces programmed cell death in the FasR-carrying target cell. Fas ligand/receptor interactions play an important role in the regulation of the immune system and the progression of cancer.
Lymphoproliferative disorders (LPDs) refer to a specific class of diagnoses, comprising a group of several conditions, in which lymphocytes are produced in excessive quantities. These disorders primarily present in patients who have a compromised immune system. Due to this factor, there are instances of these conditions being equated with "immunoproliferative disorders"; although, in terms of nomenclature, lymphoproliferative disorders are a subclass of immunoproliferative disorders—along with hypergammaglobulinemia and paraproteinemias.

In hematology, hemophagocytic lymphohistiocytosis (HLH), also known as haemophagocytic lymphohistiocytosis, and hemophagocytic or haemophagocytic syndrome, is an uncommon hematologic disorder seen more often in children than in adults. It is a life-threatening disease of severe hyperinflammation caused by uncontrolled proliferation of benign lymphocytes and macrophages that secrete high amounts of inflammatory cytokines. It is classified as one of the cytokine storm syndromes. There are inherited and non-inherited (acquired) causes of HLH.
Large granular lymphocytic (LGL) leukemia is a chronic lymphoproliferative disorder that exhibits an unexplained, chronic elevation in large granular lymphocytes (LGLs) in the peripheral blood.

WHIM syndrome is a rare congenital immunodeficiency disorder characterized by chronic noncyclic neutropenia.

B-cell prolymphocytic leukemia, referred to as B-PLL, is a rare blood cancer. It is a more aggressive, but still treatable, form of leukemia.
X-linked lymphoproliferative disease is a lymphoproliferative disorder, usually caused by SH2DIA gene mutations in males. XLP-positive individuals experience immune system deficiencies that render them unable to effectively respond to the Epstein-Barr virus (EBV), a common virus in humans that typically induces mild symptoms or infectious mononucleosis (IM) in patients. There are two currently known variations of the disorder, known as XLP1 and XLP2. XLP1 is estimated to occur in approximately one in every million males, while XLP2 is rarer, estimated to occur in one of every five million males. Due to therapies such as chemotherapy and stem cell transplants, the survival rate of XLP1 has increased dramatically since its discovery in the 1970s.

Marginal zone B-cell lymphomas, also known as marginal zone lymphomas (MZLs), are a heterogeneous group of lymphomas that derive from the malignant transformation of marginal zone B-cells. Marginal zone B cells are innate lymphoid cells that normally function by rapidly mounting IgM antibody immune responses to antigens such as those presented by infectious agents and damaged tissues. They are lymphocytes of the B-cell line that originate and mature in secondary lymphoid follicles and then move to the marginal zones of mucosa-associated lymphoid tissue, the spleen, or lymph nodes. Mucosa-associated lymphoid tissue is a diffuse system of small concentrations of lymphoid tissue found in various submucosal membrane sites of the body such as the gastrointestinal tract, mouth, nasal cavity, pharynx, thyroid gland, breast, lung, salivary glands, eye, skin and the human spleen.
Monoclonal B-cell lymphocytosis (MBL) is an asymptomatic condition in which individuals have increased blood levels of particular subtypes of monoclonal lymphocytes. This increase must persist for at least 3 months. The lymphocyte subtypes are B-cells that share certain features with the abnormal clones of lymphocytes that circulate in chronic lymphocytic leukemia/small lymphocyte lymphoma (CLL/SLL) or, less frequently, other types of B-cell malignancies. Some individuals with these circulating B-cells develop CLL/SLL or the lymphoma types indicated by their circulating monoclonal B-cells. Hence, MBL is a premalignant disorder

The death domain (DD) is a protein interaction module composed of a bundle of six alpha-helices. DD is a subclass of protein motif known as the death fold and is related in sequence and structure to the death effector domain (DED) and the caspase recruitment domain (CARD), which work in similar pathways and show similar interaction properties. DD bind each other forming oligomers. Mammals have numerous and diverse DD-containing proteins. Within these proteins, the DD domains can be found in combination with other domains, including: CARDs, DEDs, ankyrin repeats, caspase-like folds, kinase domains, leucine zippers, leucine-rich repeats (LRR), TIR domains, and ZU5 domains.
PASLI disease is a rare genetic disorder of the immune system. PASLI stands for “p110 delta activating mutation causing senescent T cells, lymphadenopathy, and immunodeficiency.” The immunodeficiency manifests as recurrent infections usually starting in childhood. These include bacterial infections of the respiratory system and chronic viremia due to Epstein–Barr virus (EBV) and/or cytomegalovirus (CMV). Individuals with PASLI disease also have an increased risk of EBV-associated lymphoma. Investigators Carrie Lucas, Michael Lenardo, and Gulbu Uzel at the National Institute of Allergy and Infectious Diseases at the U.S. National Institutes of Health and Sergey Nejentsev at the University of Cambridge, UK simultaneously described a mutation causing this condition, which they called activated PI3K delta syndrome (APDS).

RAS-associated autoimmune leukoproliferative disorder (RALD) is a rare genetic disorder of the immune system. RALD is characterized by lymphadenopathy, splenomegaly, autoimmunity, and elevation in granulocytes and monocytes. It shares many features with autoimmune lymphoproliferative syndrome and is caused by somatic mutations in NRAS or KRAS. This was first described by investigators João Oliveira and Michael Lenardo from the National Institutes of Health.
STAT3 GOF is a rare genetic disorder of the immune system. Signal transducer and activator of transcription 3 (STAT3) is a transcription factor which is encoded by the STAT3 gene in humans. Germline gain-of-function (GOF) mutations in the gene STAT3 causes this early-onset autoimmune disease characterized by lymphadenopathy, autoimmune cytopenias, multiorgan autoimmunity, infections, eczema, and short stature. Investigations conducted by Sarah E Flanagan and Mark Russell from the Institute of Biomedical and Clinical Science, University of Exeter Medical School, Emma Haapaniemi from the Institute of Biomedical and Clinical Science, University of Exeter Medical Schoolby, and Joshua Milner from the National Institute of Allergy and Infectious Disease, National Institutes of Health have described this condition in 19 patients.

Stephen E. Straus was an American physician, immunologist, virologist and science administrator. He is particularly known for his research into human herpesviruses and chronic fatigue syndrome, and for his discovery of the autoimmune lymphoproliferative syndrome genetic disorder. He headed the Laboratory of Clinical Investigation of the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH), and served as the founding director of the NIH's National Center for Complementary and Alternative Medicine.










