Distillation, also classical distillation, is the process of separating the component substances of a liquid mixture of two or more chemically discrete substances; the separation process is realized by way of the selective boiling of the mixture and the condensation of the vapors in a still.

Ethanol is an organic compound with the chemical formula CH3CH2OH. It is an alcohol, with its formula also written as C2H5OH, C2H6O or EtOH, where Et stands for ethyl. Ethanol is a volatile, flammable, colorless liquid with a characteristic wine-like odor and pungent taste. In nature, grape-sugar breaks up by the action of fermentation into alcohol or carbonic acid, without anything being added. As a psychoactive depressant, it is the active ingredient in alcoholic beverages, and the second most consumed drug globally behind caffeine.
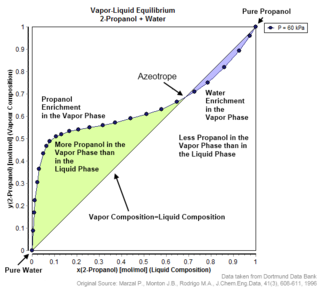
An azeotrope or a constant heating point mixture is a mixture of two or more liquids whose proportions cannot be changed by simple distillation. This happens because when an azeotrope is boiled, the vapour has the same proportions of constituents as the unboiled mixture. Knowing an azeotrope's behavior is important for distillation.

Moonshine is high-proof liquor, traditionally made or distributed illegally. The name was derived from a tradition of distilling the alcohol at night to avoid detection. In the first decades of the 21st century, commercial distilleries have adopted the term for its outlaw cachet and begun producing their own legal "moonshine", including many novelty flavored varieties, that are said to continue the tradition by using a similar method and/or locale of production.
Fractional distillation is the separation of a mixture into its component parts, or fractions. Chemical compounds are separated by heating them to a temperature at which one or more fractions of the mixture will vaporize. It uses distillation to fractionate. Generally the component parts have boiling points that differ by less than 25 °C (45 °F) from each other under a pressure of one atmosphere. If the difference in boiling points is greater than 25 °C, a simple distillation is typically used.
Pervaporation is a processing method for the separation of mixtures of liquids by partial vaporization through a non-porous or porous membrane.
A desiccant is a hygroscopic substance that is used to induce or sustain a state of dryness (desiccation) in its vicinity; it is the opposite of a humectant. Commonly encountered pre-packaged desiccants are solids that absorb water. Desiccants for specialized purposes may be in forms other than solid, and may work through other principles, such as chemical bonding of water molecules. They are commonly encountered in foods to retain crispness. Industrially, desiccants are widely used to control the level of water in gas streams.

A column still, also called a continuous still, patent still or Coffey still, is a variety of still consisting of two columns. Column stills can produce rectified spirit.

Extractive distillation is defined as distillation in the presence of a miscible, high-boiling, relatively non-volatile component, the solvent, that forms no azeotrope with the other components in the mixture. The method is used for mixtures having a low value of relative volatility, nearing unity. Such mixtures cannot be separated by simple distillation, because the volatility of the two components in the mixture is nearly the same, causing them to evaporate at nearly the same temperature at a similar rate, making normal distillation impractical.

Steam distillation is a separation process that consists of distilling water together with other volatile and non-volatile components. The steam from the boiling water carries the vapor of the volatiles to a condenser; both are cooled and return to the liquid or solid state, while the non-volatile residues remain behind in the boiling container.

A molecular sieve is a material with pores of uniform size which link the interior of the solid to its exterior. These materials embody the molecular sieve effect: "With respect to porous solids, the surface associated with pores communicating with the outside space may be called the internal surface. Because the accessibility of pores may depend on the size of the fluid molecules, the extent of the internal surface may depend on the size of the molecules comprising the fluid, and may be different for the various components of a fluid mixture." The specification for the pores is that they not only communicate from the exterior to the interior, but the pores are uniform in size. Many kinds of materials exhibit some molecular sieves, but zeolites dominate the field. Zeolites are almost always aluminosilicates, or variants where some or all of the Si or Al centers are replaced by similarly charged elements.
Rectified spirit, also known as neutral spirits, rectified alcohol or ethyl alcohol of agricultural origin, is highly concentrated ethanol that has been purified by means of repeated distillation in a process called rectification. In some countries, denatured alcohol or denatured rectified spirit may commonly be available as "rectified spirit", because in some countries the retail sale of rectified alcohol in its non-denatured form is prohibited.
Reactive distillation is a process where the chemical reactor is also the still. Separation of the product from the reaction mixture does not need a separate distillation step which saves energy and materials. This technique can be useful for equilibrium-limited reactions such as esterification and ester hydrolysis reactions. Conversion can be increased beyond what is expected by the equilibrium due to the continuous removal of reaction products from the reactive zone. This approach can also reduce capital and investment costs.
The Marcusson apparatus, Dean-Stark apparatus, Dean–Stark receiver, distilling trap, or Dean–Stark Head is a piece of laboratory glassware used in synthetic chemistry to collect water from a reactor. It is used in combination with a reflux condenser and a distillation flask for the separation of water from liquids. This may be a continuous removal of the water that is produced during a chemical reaction performed at reflux temperature, such as in esterification reactions. The original setup by Julius Marcusson was refined by the American chemists Ernest Woodward Dean (1888–1959) and David Dewey Stark (1893–1979) in 1920 for determination of the water content in petroleum.
A zeotropicmixture, or non-azeotropic mixture, is a mixture with liquid components that have different boiling points. For example, nitrogen, methane, ethane, propane, and isobutane constitute a zeotropic mixture. Individual substances within the mixture do not evaporate or condense at the same temperature as one substance. In other words, the mixture has a temperature glide, as the phase change occurs in a temperature range of about four to seven degrees Celsius, rather than at a constant temperature. On temperature-composition graphs, this temperature glide can be seen as the temperature difference between the bubble point and dew point. For zeotropic mixtures, the temperatures on the bubble (boiling) curve are between the individual component's boiling temperatures. When a zeotropic mixture is boiled or condensed, the composition of the liquid and the vapor changes according to the mixtures's temperature-composition diagram.
Batch distillation refers to the use of distillation in batches, meaning that a mixture is distilled to separate it into its component fractions before the distillation still is again charged with more mixture and the process is repeated. This is in contrast with continuous distillation where the feedstock is added and the distillate drawn off without interruption. Batch distillation has always been an important part of the production of seasonal, or low capacity and high-purity chemicals. It is a very frequent separation process in the pharmaceutical industry.
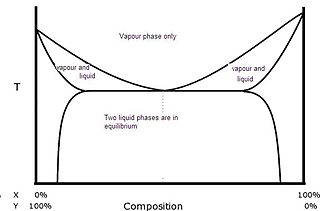
A heteroazeotrope is an azeotrope where the vapour phase coexists with two liquid phases. Sketch of a T-x/y equilibrium curve of a typical heteroazeotropic mixture
This page contains tables of azeotrope data for various binary and ternary mixtures of solvents. The data include the composition of a mixture by weight, the boiling point (b.p.) of a component, the boiling point of a mixture, and the specific gravity of the mixture. Boiling points are reported at a pressure of 760 mm Hg unless otherwise stated. Where the mixture separates into layers, values are shown for upper (U) and lower (L) layers.

2-Methyltetrahydrofuran (2-MeTHF) is an organic compound with the molecular formula C5H10O. It is a highly flammable, mobile liquid. It is mainly used as a replacement for Tetrahydrofuran (THF) in specialized applications for its better performance, such as to obtain higher reaction temperatures, or easier separations (as, unlike THF, it is not miscible with water). It is derived from sugars via furfural and is occasionally touted as a biofuel.

A residue curve describes the change in the composition of the liquid phase of a chemical mixture during continuous evaporation at the condition of vapor–liquid equilibrium. Multiple residue curves for a single system are called residue curves map.