Barrelene Last updated August 16, 2025 Barrelene Names Preferred IUPAC name Bicyclo[2.2.2]octa-2,5,7-triene
[ 1] Identifiers ChemSpider UNII InChI=1S/C8H8/c1-2-8-5-3-7(1)4-6-8/h1-8H
Y Key:
RHCCUQVVABYRDN-UHFFFAOYSA-N
Y InChI=1/C8H8/c1-2-8-5-3-7(1)4-6-8/h1-8H
Key: RHCCUQVVABYRDN-UHFFFAOYAN
C1(C=C2)C=CC2C=C1
C\1=C\C\2/C=C\C/1/C=C/2
Properties C 8 H 8 Molar mass 104.15 Density 1.013 g/mL Boiling point 153.7 °C (308.7 °F; 426.8 K) Except where otherwise noted, data are given for materials in their
standard state (at 25
°C [77
°F], 100
kPa).
Chemical compound
Barrelene is a bicyclic organic compound with chemical formula C8 H8 and systematic name bicyclo[2.2.2]octa-2,5,7-triene. First synthesized and described by Howard Zimmerman in 1960, the name derives from the resemblance to a barrel , with the staves being three ethylene units attached to two methine groups. It is the formal Diels–Alder adduct of benzene and acetylene . Due to its unusual molecular geometry , the compound is of considerable interest to theoretical chemists.
Iptycenes , with the alkene groups part of an arenes , are related compounds. It is also a starting material for many other organic compounds, such as semibullvalene .
Synthesis The original Zimmerman synthesis modified in 1969 [ 2] starts from coumalic acid : [ note 1]
The synthesis of barrelene as reported by Zimmermann in 1969.
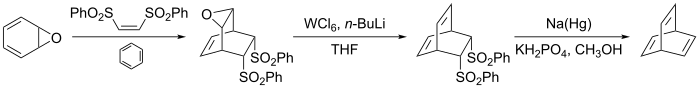
Many alternative routes have been devised since then, one of them starting from benzene oxide : [ 3] [ 4]
An alternate route that allows synthesis of the parent barrelene system and a variety of substituted barrelenes has also been reported. [ 5]
References ↑ International Union of Pure and Applied Chemistry (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013 . The Royal Society of Chemistry . p. 1257. doi :10.1039/9781849733069 . ISBN 978-0-85404-182-4 ↑ Zimmerman, Howard E.; Grunewald, Gary L.; Paufler, Robert M.; Sherwin, Maynard A. (April 1969). "Synthesis and physical properties of barrelene, a unique Moebius-like molecule" . Journal of the American Chemical Society . 91 (9): 2330– 2338. doi :10.1021/ja01037a024 . ISSN 0002-7863 . ↑ Cossu, Sergio; Battaggia, Simone; De Lucchi, Ottorino (1997-06-13). "Barrelene, a New Convenient Synthesis" . The Journal of Organic Chemistry . 62 (12): 4162– 4163. doi :10.1021/jo962267f . ISSN 0022-3263 . ↑ Step one in this reaction between oxepin (one of the possible tautomers ) with (Z )-1,2-bis(phenylsulfonyl)ethylene is a Diels–Alder reaction . The reagents for de-epoxidation are tungsten hexachloride and butyllithium . The second elimination reaction takes place with sodium amalgam in Julia olefination style. ↑ Wagaman, Michael W.; Bellmann, Erika; Cucullu, Michèle; Grubbs, Robert H. (1997-12-01). "Synthesis of Substituted Bicyclo[ 2.2.2] octatrienes" . The Journal of Organic Chemistry . 62 (26): 9076– 9082. doi :10.1021/jo971039y . ISSN 0022-3263 . ↑ endo, exo,syn-3,7,10-Trioxapentacyclo[3.3.3.02,4 .06,8 .09,11 ]undecane ↑ Kozhushkov, Sergei I.; Preuß, Thomas; Yufit, Dmitrii S.; Howard, Judith A. K.; Meindl, Kathrin; Rühl, Stephan; Yamamoto, Chiyo; Okamoto, Yoshio; Schreiner, Peter R.; Rinderspacher, B. Christopher; de Meijere, Armin (June 2006). "4,7,11‐Triheterotrishomocubanes – Propeller‐Shaped Highly Symmetrical Chiral Molecules Derived from Barrelene" . European Journal of Organic Chemistry . 2006 (11): 2590– 2600. doi :10.1002/ejoc.200600019 . ISSN 1434-193X . ↑ Pu, Lin; Wagaman, Michael W.; Grubbs, Robert H. (1996-01-01). "Synthesis of Poly(1,4-naphthylenevinylenes): Metathesis Polymerization of Benzobarrelenes" . Macromolecules . 29 (4): 1138– 1143. doi :10.1021/ma9500143 . ISSN 0024-9297 . ↑ Wagaman, Michael W.; Grubbs, Robert H. (1997-07-01). "Synthesis of Organic and Water Soluble Poly(1,4-phenylenevinylenes) Containing Carboxyl Groups: Living Ring-Opening Metathesis Polymerization (ROMP) of 2,3-Dicarboxybarrelenes" . Macromolecules . 30 (14): 3978– 3985. doi :10.1021/ma9701595 . ISSN 0024-9297 . ↑ Zimmerman, H. E.; Grunewald, G. L. (1966). "The Chemistry of Barrelene. III. A Unique Photoisomerization to Semibullvalene". J. Am. Chem. Soc. 88 (1): 183– 184. doi :10.1021/ja00953a045 . This page is based on this
Wikipedia article Text is available under the
CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.