
Pentazocine, sold under the brand name Talwin among others, is a painkiller used to treat moderate to severe pain. It is believed to work by activating (agonizing) κ-opioid receptors (KOR) and blocking (antagonizing) μ-opioid receptors (MOR). As such it is called an opioid as it delivers its effects on pain by interacting with the opioid receptors. It shares many of the side effects of other opioids like constipation, nausea, itching, drowsiness and respiratory depression, but unlike most other opioids it fairly frequently causes hallucinations, nightmares and delusions. It is also, unlike most other opioids, subject to a ceiling effect, which is when at a certain dose no more pain relief, or side effects, is obtained by increasing the dose any further.

Nalbuphine, sold under the brand names Nubain among others, is an opioid analgesic which is used in the treatment of pain. It is given by injection into a vein, muscle, or fat.
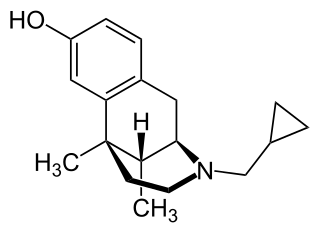
Cyclazocine is a mixed opioid agonist/antagonist related to dezocine, pentazocine and phenazocine. This family of opioid drugs is called the benzomorphans or benzazocines. It is a KOR agonist and MOR partial agonist, and also has high affinity for the DOR.
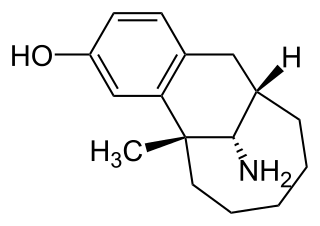
Dezocine is a marketed opioid analgesic of the benzomorphan group. First synthesized in 1970, it acts as a modulator of mu-, delta-, and kappa-opioid receptors. Dezocine is a mixed agonist/antagonist of opioid receptors. It is related to other benzomorphans such as pentazocine, with a similar profile of effects that include analgesia and euphoria. Unlike many other benzomorphans however, it is a silent antagonist of the κ-opioid receptor, and in accordance, does not produce side effects such as dysphoria or hallucinations at any dose.

Metazocine is an opioid analgesic related to pentazocine. While metazocine has significant analgesic effects, mediated through a mixed agonist–antagonist action at the mu opioid receptor, its clinical use is limited by dysphoric and hallucinogenic effects which are most likely caused by activity at kappa opioid receptors and/or sigma receptors.

Phenazocine is an opioid analgesic drug, which is related to pentazocine and has a similar profile of effects.
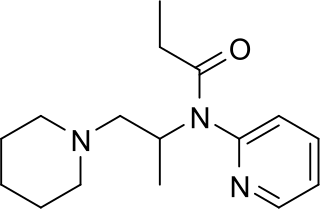
Propiram is a partial mu opioid receptor agonist and weak mu antagonist analgesic from the ampromide family of drugs related to other drugs such as phenampromide and diampromide. It was invented in 1963 in the United Kingdom by Bayer but was not widely marketed, although it saw some limited clinical use, especially in dentistry. Propiram reached Phase III clinical trials in the United States and Canada.

L-838,417 is an anxiolytic drug used in scientific research. It has similar effects to benzodiazepine drugs, but is structurally distinct and so is classed as a nonbenzodiazepine anxiolytic. The compound was developed by Merck, Sharp and Dohme.
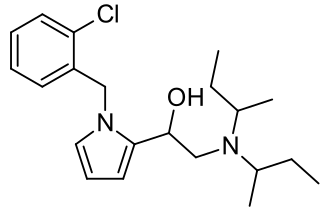
Viminol is an opioid analgesic developed by a team at the drug company Zambon in the 1960s. Viminol is based on the α-pyrryl-2-aminoethanol structure, unlike any other class of opioids.

Levophenacylmorphan is a morphinan derivative that acts as an opioid agonist. It has potent analgesic effects and is around 10x more potent than morphine. Adverse effects associated with its use are those of the opioids as a whole, including pruritus, nausea, respiratory depression, euphoria and development of tolerance and dependence to its effects.

Tebanicline is a potent synthetic nicotinic (non-opioid) analgesic drug developed by Abbott. It was developed as a less toxic analogue of the potent poison dart frog-derived compound epibatidine, which is some 200x stronger than morphine as an analgesic but produces extremely dangerous toxic side effects. Like epibatidine, tebanicline showed potent analgesic activity against neuropathic pain in both animal and human trials, but with far less toxicity than its parent compound. It acts as a partial agonist at neuronal nicotinic acetylcholine receptors, binding to both the α3β4 and the α4β2 subtypes.
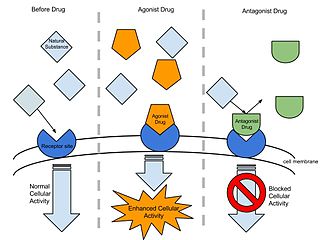
In pharmacology the term agonist-antagonist or mixed agonist/antagonist is used to refer to a drug which under some conditions behaves as an agonist while under other conditions, behaves as an antagonist.

Alazocine, also known more commonly as N-allylnormetazocine (NANM), is a synthetic opioid analgesic of the benzomorphan family related to metazocine, which was never marketed. In addition to its opioid activity, the drug is a sigma receptor agonist, and has been used widely in scientific research in studies of this receptor. Alazocine is described as a potent analgesic, psychotomimetic or hallucinogen, and morphine or opioid antagonist. Moreover, one of its enantiomers was the first compound that was found to selectively label the σ1 receptor, and led to the discovery and characterization of the receptor.

Bremazocine is a κ-opioid receptor agonist related to pentazocine. It has potent and long-lasting analgesic and diuretic effects. It has 200 times the activity of morphine, but appears to have no addictive properties and does not depress breathing. The crystal structure of bremazocine was determined in 1984

AD-1211 is an opioid analgesic drug invented in the 1970s by Dainippon Pharmaceutical Co. It is chemically a 1-substituted-4-prenyl-piperazine derivative, which is structurally unrelated to most other opioid drugs. The (S)-enantiomers in this series are more active as opioid agonists, but the less active (R)-enantiomer of this compound, AD-1211, is a mixed agonist–antagonist at opioid receptors with a similar pharmacological profile to pentazocine, and has atypical opioid effects with little development of tolerance or dependence seen after extended administration in animal studies.

8-Carboxamidocyclazocine (8-CAC) is an opioid analgesic drug related to cyclazocine, discovered by medicinal chemist Mark P. Wentland and co-workers in Cogswell Laboratory at Rensselaer Polytechnic Institute. Similarly to cyclazocine, 8-CAC acts as an agonist at both the μ and κ opioid receptors, but has a much longer duration of action than cyclazocine, and does not have μ antagonist activity. Unexpectedly it was discovered that the phenolic hydroxyl group of cyclazocine could be replaced by a carboxamido group with only slight loss of potency at opioid receptors, and this discovery has subsequently been used to develop many novel opioid derivatives where the phenolic hydroxy group has been replaced by either carboxamide or a variety of larger groups. Due to their strong κ-opioid agonist activity, these drugs are not suited for use as analgesics in humans, but have instead been researched as potential drugs for the treatment of cocaine addiction.

Fluorophen, or fluorofen, is a fluorinated analogue of phenazocine, an opioid drug of the benzomorphan group, which was developed as a radioligand for the purpose of labeling opioid receptors during PET scans. Unlike most other benzomorphan derivatives, fluorophen acts as a full agonist of the opioid receptors with preferential affinity for the μ-opioid receptor, similar but slightly lower affinity for the δ-opioid receptor, and very low affinity for the κ-opioid receptor.

Nalmexone (INN), or nalmexone hydrochloride (USAN), is a semisynthetic, opioid partial agonist or mixed agonist-antagonist with both analgesic and narcotic antagonist properties that was never marketed. In clinical studies it was found to have comparable analgesic efficacy to morphine, though with several-fold reduced potency. In addition, nalmexone's side effects, the most common of which were sleepiness and sweating, were reported to be similar to those of morphine, albeit with a noticeably higher degree of incidence.

Difelikefalin (INN), also known as D-Phe-D-Phe-D-Leu-D-Lys-[γ-(4-N-piperidinyl)amino carboxylic acid], is an analgesic opioid peptide acting as a peripherally specific, highly selective agonist of the κ-opioid receptor (KOR). It is under development by Cara Therapeutics as an intravenous agent for the treatment of postoperative pain. An oral formulation has also been developed. Due to its peripheral selectivity, difelikefalin lacks the central side effects like sedation, dysphoria, and hallucinations of previous KOR-acting analgesics such as pentazocine and phenazocine. In addition to use as an analgesic, difelikefalin is also being investigated for the treatment of pruritus (itching). Difelikefalin has completed phase II clinical trials for postoperative pain and has demonstrated significant and "robust" clinical efficacy, along with being safe and well tolerated. It has also completed a phase III clinical trial for uremic pruritus in hemodialysis patients.
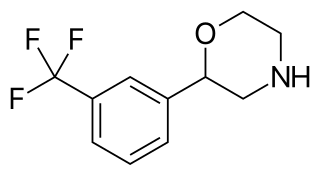
Flumexadol (INN) is a drug described and researched as a non-opioid analgesic which was never marketed. It has been found to act as an agonist of the serotonin 5-HT1A and 5-HT2C receptors and, to a much lesser extent, of the 5-HT2A receptor. According to Nilsson (2006) in a paper on 5-HT2C receptor agonists as potential anorectics, "The (+)-enantiomer of this compound showed [...] affinity for the 5-HT2C receptor (Ki) 25 nM) [...] and was 40-fold selective over the 5-HT2A receptor in receptor binding studies. Curiously, the racemic version [...], also known as 1841 CERM, was originally reported to possess analgesic properties while no association with 5-HT2C receptor activity was mentioned." It is implied that flumexadol might be employable as an anorectic in addition to analgesic. Though flumexadol itself has never been approved for medical use, oxaflozane is a prodrug of the compound that was formerly used clinically in France as an antidepressant and anxiolytic agent.




















