
In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of one or more hydroxyl groups (—OH) bonded directly to an aromatic hydrocarbon group. The simplest is phenol, C
6H
5OH. Phenolic compounds are classified as simple phenols or polyphenols based on the number of phenol units in the molecule.
The quinones are a class of organic compounds that are formally "derived from aromatic compounds [such as benzene or naphthalene] by conversion of an even number of –CH= groups into –C(=O)– groups with any necessary rearrangement of double bonds, resulting in "a fully conjugated cyclic dione structure". The archetypical member of the class is 1,4-benzoquinone or cyclohexadienedione, often called simply "quinone". Other important examples are 1,2-benzoquinone (ortho-quinone), 1,4-naphthoquinone and 9,10-anthraquinone.

1,4-Benzoquinone, commonly known as para-quinone, is a chemical compound with the formula C6H4O2. In a pure state, it forms bright-yellow crystals with a characteristic irritating odor, resembling that of chlorine, bleach, and hot plastic or formaldehyde. This six-membered ring compound is the oxidized derivative of 1,4-hydroquinone. The molecule is multifunctional: it exhibits properties of a ketone, being able to form oximes; an oxidant, forming the dihydroxy derivative; and an alkene, undergoing addition reactions, especially those typical for α,β-unsaturated ketones. 1,4-Benzoquinone is sensitive toward both strong mineral acids and alkali, which cause condensation and decomposition of the compound.
1,2-Benzoquinone, also called ortho-benzoquinone, is an organic compound with formula C6H4O2. It is one of the two isomers of quinone, the other being 1,4-benzoquinone. It is a red volatile solid that is soluble in water and ethyl ether. It is rarely encountered because of its instability, but it is of fundamental interest as the parent compound of many derivatives which are known.
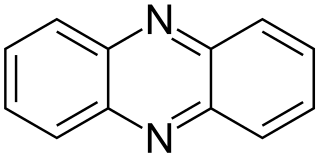
Phenazine is an organic compound with the formula (C6H4)2N2. It is a dibenzo annulated pyrazine, and the parent substance of many dyestuffs, such as the toluylene red, indulines, and safranines (and the closely related eurhodines). Phenazine crystallizes in yellow needles, which are only sparingly soluble in alcohol. Sulfuric acid dissolves it, forming a deep-red solution.
Catechol oxidase is a copper oxidase that contains a type 3 di-copper cofactor and catalyzes the oxidation of ortho-diphenols into ortho-quinones coupled with the reduction of molecular oxygen to water. It is present in a variety of species of plants and fungi including Ipomoea batatas and Camellia sinensis. Metalloenzymes with type 3 copper centers are characterized by their ability to reversibly bind dioxygen at ambient conditions. In plants, catechol oxidase plays a key role in enzymatic browning by catalyzing the oxidation of catechol to o-quinone in the presence of oxygen, which can rapidly polymerize to form the melanin that grants damaged fruits their dark brown coloration.

The Hofmann–Martius rearrangement in organic chemistry is a rearrangement reaction converting an N-alkylated aniline to the corresponding ortho and / or para aryl-alkylated aniline. The reaction requires heat, and the catalyst is an acid like hydrochloric acid.
Polymer stabilizers are chemical additives which may be added to polymeric materials, such as plastics, to inhibit or retard their degradation. Common polymer degradation processes include oxidation, UV-damage, thermal degradation, ozonolysis, combinations thereof such as photo-oxidation, as well as reactions with catalyst residues, dyes, or impurities. All of these degrade the polymer at a chemical level, via chain scission, uncontrolled recombination and cross-linking, which adversely affects many key properties such as strength, malleability, appearance and colour.

Chloranil is a quinone with the molecular formula C6Cl4O2. Also known as tetrachloro-1,4-benzoquinone, it is a yellow solid. Like the parent benzoquinone, chloranil is a planar molecule that functions as a mild oxidant.

Irigenin is an O-methylated isoflavone, a type of flavonoid. It can be isolated from the rhizomes of the leopard lily, and Iris kemaonensis.
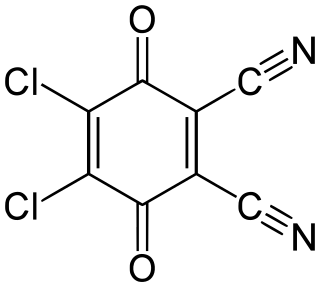
2,3-Dichloro-5,6-dicyano-1,4-benzoquinone (or DDQ) is the chemical reagent with formula C6Cl2(CN)2O2. This oxidant is useful for the dehydrogenation of alcohols, phenols, and steroid ketones in organic chemistry. DDQ decomposes in water, but is stable in aqueous mineral acid.
The molecular formula C6H4O2 (molar mass: 108.09 g/mol) may refer to:
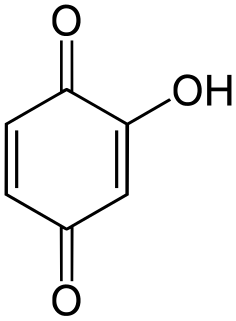
Hydroxyquinone often refers to a hydroxybenzoquinone, any organic compound with formula C
6H
4O
3 which can be viewed as a derivative of a benzoquinone through replacement of one hydrogen atom (H) by a hydroxyl group (-OH). When unqualified, the terms usually mean specifically the compound 2-hydroxy-1,4-benzoquinone, derived from 1,4-benzoquinone or para-benzoquinone.
A hydroxybenzoquinone is any of several organic compounds that can be viewed as derivatives of a benzoquinone through replacement of one hydrogen atom (H) by a hydroxyl group (-OH).
Phenol oxidation with hypervalent iodine reagents leads to the formation of quinone-type products or iodonium ylides, depending on the structure of the phenol. Trapping of either product is possible with a suitable reagent, and this method is often employed in tandem with a second process.

A quinone methide is a type of conjugated organic compound that contain a cyclohexadiene with a carbonyl and an exocyclic methylidene or extended alkene unit. It is analogous to a quinone, but having one of the double bonded oxygens replaced with a carbon. The carbonyl and methylidene are usually oriented either ortho or para to each other. There are some examples of transient synthetic meta quinone methides.

Xylylene (sometimes quinone-dimethides) comprises two isomeric organic compounds with the formula C6H4(CH2)2. These compounds are related to the corresponding quinones and quinone methides by replacement of the oxygen atoms by CH2 groups. ortho- and para-xylylene are best known, although neither is stable in solid or liquid form. The meta form is a diradical. Certain substituted derivatives of xylylenes are however highly stable, an example being tetracyanoquinodimethane.
Iris bungei is a beardless iris in the genus Iris, in the subgenus Limniris and in the series Tenuifoliae of the genus. It is a rhizomatous herbaceous perennial, from Mongolia, Tibet and China. It has green leaves, short stem and 2 violet, purple, lavender or blue flowers.

Iris potaninii is a species in the genus Iris, it is also in the subgenus of Iris and in the Psammiris section. It is a rhizomatous perennial, from Siberia in Russia, Mongolia and China. It is a dwarf plant, having either subterranean or very small stems, long thin leaves and yellow, or dark violet to purplish blue flowers. It is cultivated as an ornamental plant in temperate regions.

Iris kemaonensis, the Kumaon iris, is a plant species in the genus Iris, it is also in the subgenus Iris and in the section Pseudoregelia. It is a rhizomatous perennial, from Tibetan China, Bhutan, India, Kashmir and Nepal. It has light green or yellowish green leaves, that extend after flowering time. It has a short stem, 1–2 fragrant flowers that are purple, lilac, lilac-purple or pale purple. They also have darker coloured blotches or spots. It is cultivated as an ornamental plant in temperate regions. It is often known as Iris kumaonensis, due to a publishing error.












