


The benzylisoquinoline alkaloids are natural products that can be classified as isoquinoline alkaloidss and are derived from benzylisoquinoline. They also include the benzyl(tetrahydro)isoquinoline alkaloids.



The benzylisoquinoline alkaloids are natural products that can be classified as isoquinoline alkaloidss and are derived from benzylisoquinoline. They also include the benzyl(tetrahydro)isoquinoline alkaloids.
Benzylisoquinoline alkaloids are found in several plant families, including the poppy family (Papaveraceae), the annonaceae family, and the laurel family. [1] Well-known representatives are primarily found in poppy plants, specifically those from which opium is derived, as well as in actaea. [2] For instance, reticuline has been isolated from Annona reticulata. [3]
Over 2500 biologically active derivatives are known from the benzylisoquinoline alkaloids. [4] Based on their structure, the compounds can be divided into numerous subgroups: the aporphines, the phthalideisoquinoline alkaloids, the morphinans, the protoberberine alkaloids, and the pavins. [5]
Among the known individual substances in this group are papaverine. Additional examples of compounds in this group are the benzyltetrahydroisoquinoline alkaloids reticuline and laudanosine. [1]
Papaverine has vasodilator and muscle relaxant properties. [3] Laudanosine acts as a tetanic poison. [1]
The biosynthesis of benzylisoquinoline alkaloids has been intensively studied. It begins with the amino acid tyrosine, which is converted to dopamine by hydroxylation and decarboxylation and to 4-hydroxyphenylacetaldehyde by oxidative deamination, respectively. These two compounds are converted into dopamine by enzyme Norcoclaurine synthase catalyzed condensation reaction to form the benzylisoquinoline backbone. [6]

The benzylisoquinoline from this reaction may have different substituents, [6] reticulin is an important intermediate. [5]

Alkaloids are a class of basic, naturally occurring organic compounds that contain at least one nitrogen atom. This group also includes some related compounds with neutral and even weakly acidic properties. Some synthetic compounds of similar structure may also be termed alkaloids. In addition to carbon, hydrogen and nitrogen, alkaloids may also contain oxygen or sulfur. More rarely still, they may contain elements such as phosphorus, chlorine, and bromine.

Papaver somniferum, commonly known as the opium poppy or breadseed poppy, is a species of flowering plant in the family Papaveraceae. It is the species of plant from which both opium and poppy seeds are derived and is also a valuable ornamental plant grown in gardens. Its native range was east of the Mediterranean Sea, but now is obscured by ancient introductions and cultivation, being naturalized across much of Europe and Asia.

Isoquinoline is a heterocyclic aromatic organic compound. It is a structural isomer of quinoline. Isoquinoline and quinoline are benzopyridines, which are composed of a benzene ring fused to a pyridine ring. In a broader sense, the term isoquinoline is used to make reference to isoquinoline derivatives. 1-Benzylisoquinoline is the structural backbone in naturally occurring alkaloids including papaverine. The isoquinoline ring in these natural compound derives from the aromatic amino acid tyrosine.

Noscapine is a benzylisoquinoline alkaloid, of the phthalideisoquinoline structural subgroup, which has been isolated from numerous species of the family Papaveraceae. It lacks significant hypnotic, euphoric, or analgesic effects affording it with very low addictive potential. This agent is primarily used for its antitussive (cough-suppressing) effects.

Sanguinaria canadensis, bloodroot, is a perennial, herbaceous flowering plant native to eastern North America. It is the only species in the genus Sanguinaria, included in the poppy family Papaveraceae, and is most closely related to Eomecon of eastern Asia.

Sanguinarine is a polycyclic quaternary alkaloid. It is extracted from some plants, including the bloodroot plant, from whose scientific name, Sanguinaria canadensis, its name is derived; the Mexican prickly poppy ; Chelidonium majus; and Macleaya cordata.

Berberine is a quaternary ammonium salt from the protoberberine group of benzylisoquinoline alkaloids
The enzyme (S)-norcoclaurine synthase (EC 4.2.1.78) catalyzes the chemical reaction

Laudanosine or N-methyltetrahydropapaverine is a recognized metabolite of atracurium and cisatracurium. Laudanosine decreases the seizure threshold, and thus it can induce seizures if present at sufficient threshold concentrations; however such concentrations are unlikely to be produced consequent to chemodegradable metabolism of clinically administered doses of cisatracurium or atracurium.

Deoxyepinephrine, also known by the common names N-methyldopamine and epinine, is an organic compound and natural product that is structurally related to the important neurotransmitters dopamine and epinephrine. All three of these compounds also belong to the catecholamine family. The pharmacology of epinine largely resembles that of its "parent", dopamine. Epinine has been found in plants, insects and animals. It is also of significance as the active metabolic breakdown product of the prodrug ibopamine, which has been used to treat congestive heart failure.

Higenamine (norcoclaurine) is a chemical compound found in a variety of plants including Nandina domestica (fruit), Aconitum carmichaelii (root), Asarum heterotropioides, Galium divaricatum, Annona squamosa, and Nelumbo nucifera.

Toxiferine is a curare toxin. It is a bisindole alkaloid derived from Strychnos toxifera and a nicotinic acetylcholine receptor antagonist. This alkaloid is the main toxic component of Calabash curare, and one of the most toxic plant alkaloids known. The lethal dose (LD50) for mice has been determined as 10 - 60 µg/kg by intravenous administration. It is a muscle relaxant that causes paralysis of skeletal muscle, which takes approximately 2 hours to recovery for a moderate dose, and 8 hours of total paralysis with a 20-fold paralytic dose. The paralysis can be antagonized by neostigmine
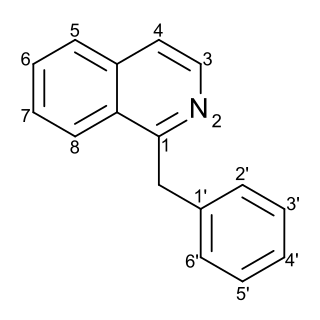
Substitution of the heterocycle isoquinoline at the C1 position by a benzyl group provides 1‑benzylisoquinoline, the most widely examined of the numerous benzylisoquinoline structural isomers. The 1-benzylisoquinoline moiety can be identified within numerous compounds of pharmaceutical interest, such as moxaverine; but most notably it is found within the structures of a wide variety of plant natural products, collectively referred to as benzylisoquinoline alkaloids. This class is exemplified in part by the following compounds: papaverine, noscapine, codeine, morphine, apomorphine, berberine, tubocurarine.

(S)-Canadine, also known as (S)-tetrahydroberberine and xanthopuccine, is a benzylisoquinoline alkaloid (BIA), of the protoberberine structural subgroup, and is present in many plants from the family Papaveraceae, such as Corydalis yanhusuo and C. turtschaninovii.

Chelidonine is an isolate of Papaveraceae with acetylcholinesterase and butyrylcholinesterase inhibitory activity.
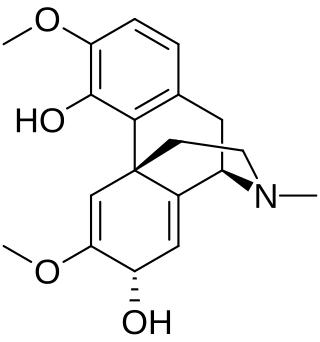
Salutaridinol is a modified benzyltetrahydroisoquinoline alkaloid with the formula C19H23NO4. It is produced in the secondary metabolism of the opium poppy Papaver somniferum (Papaveraceae) as an intermediate in the biosynthetic pathway that generates morphine. As an isoquinoline alkaloid, it is fundamentally derived from tyrosine as part of the shikimate pathway of secondary metabolism. Salutaridinol is a product of the enzyme salutaridine: NADPH 7-oxidoreductase and the substrate for the enzyme salutaridinol 7-O-acetyltransferase, which are two of the four enzymes in the morphine biosynthesis pathway that generates morphine from (R)-reticuline. Salutaridinol's unique position adjacent to two of the four enzymes in the morphine biosynthesis pathway gives it an important role in enzymatic, genetic, and synthetic biology studies of morphine biosynthesis. Salutaridinol levels are indicative of the flux through the morphine biosynthesis pathway and the efficacy of both salutaridine: NADPH 7-oxidoreductase and salutaridinol 7-O-acetyltransferase.

Berberine bridge enzyme-like form a subgroup of the superfamily of FAD-linked oxidases, structurally characterized by a typical fold observed initially for vanillyl-alcohol oxidase (VAO). This proteins are part of a multigene family (PF08031) that can be found in plants, fungi and bacteria.
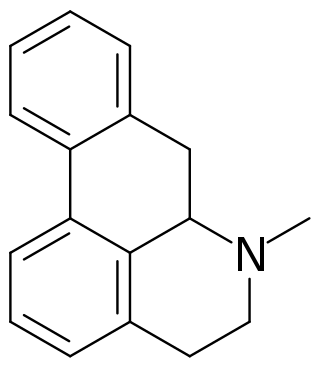
Aporphine alkaloids are naturally occurring chemical compounds from the group of alkaloids. After the benzylisoquinoline alkaloids they are the second largest group of isoquinoline alkaloids.

Isoquinoline alkaloids are natural products of the group of alkaloids, which are chemically derived from isoquinoline. They form the largest group among the alkaloids.
Bisbenzylisoquinoline alkaloids are natural products found primarily in the plant families of the barberry family, the Menispermaceae, the Monimiaceae, and the buttercup family.