
Biomedical engineering (BME) or medical engineering is the application of engineering principles and design concepts to medicine and biology for healthcare purposes. BME is also traditionally known as "bioengineering", but this term has come to also refer to biological engineering. This field seeks to close the gap between engineering and medicine, combining the design and problem-solving skills of engineering with medical biological sciences to advance health care treatment, including diagnosis, monitoring, and therapy. Also included under the scope of a biomedical engineer is the management of current medical equipment in hospitals while adhering to relevant industry standards. This involves making equipment recommendations, procurement, routine testing, and preventive maintenance, a role also known as a Biomedical Equipment Technician (BMET) or as clinical engineering.
An artificial organ is a human made organ device or tissue that is implanted or integrated into a human — interfacing with living tissue — to replace a natural organ, to duplicate or augment a specific function or functions so the patient may return to a normal life as soon as possible. The replaced function does not have to be related to life support, but it often is. For example, replacement bones and joints, such as those found in hip replacements, could also be considered artificial organs.
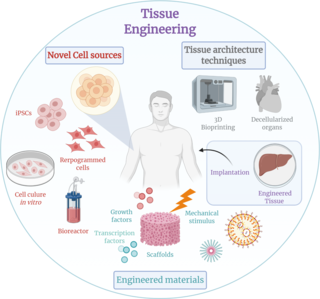
Tissue engineering is a biomedical engineering discipline that uses a combination of cells, engineering, materials methods, and suitable biochemical and physicochemical factors to restore, maintain, improve, or replace different types of biological tissues. Tissue engineering often involves the use of cells placed on tissue scaffolds in the formation of new viable tissue for a medical purpose but is not limited to applications involving cells and tissue scaffolds. While it was once categorized as a sub-field of biomaterials, having grown in scope and importance it can be considered as a field in its own.

Xenotransplantation, or heterologous transplant, is the transplantation of living cells, tissues or organs from one species to another. Such cells, tissues or organs are called xenografts or xenotransplants. It is contrasted with allotransplantation, syngeneic transplantation or isotransplantation and autotransplantation.
Allotransplant is the transplantation of cells, tissues, or organs to a recipient from a genetically non-identical donor of the same species. The transplant is called an allograft, allogeneic transplant, or homograft. Most human tissue and organ transplants are allografts.
A cord blood bank is a facility which stores umbilical cord blood for future use. Both private and public cord blood banks have developed in response to the potential for cord blood in treating diseases of the blood and immune systems. Public cord blood banks accept donations to be used for anyone in need, and as such function like public blood banks. Traditionally, public cord blood banking has been more widely accepted by the medical community. Private cord blood banks store cord blood solely for potential use by the donor or donor's family. Private banks typically charge around $2,000 for the collection and around $200 a year for storage.

A medical device is any device intended to be used for medical purposes. Significant potential for hazards are inherent when using a device for medical purposes and thus medical devices must be proved safe and effective with reasonable assurance before regulating governments allow marketing of the device in their country. As a general rule, as the associated risk of the device increases the amount of testing required to establish safety and efficacy also increases. Further, as associated risk increases the potential benefit to the patient must also increase.

An implant is a medical device manufactured to replace a missing biological structure, support a damaged biological structure, or enhance an existing biological structure. Medical implants are man-made devices, in contrast to a transplant, which is a transplanted biomedical tissue. The surface of implants that contact the body might be made of a biomedical material such as titanium, silicone, or apatite depending on what is the most functional. In some cases implants contain electronics, e.g. artificial pacemaker and cochlear implants. Some implants are bioactive, such as subcutaneous drug delivery devices in the form of implantable pills or drug-eluting stents.
A biopharmaceutical, also known as a biologic(al) medical product, or biologic, is any pharmaceutical drug product manufactured in, extracted from, or semisynthesized from biological sources. Different from totally synthesized pharmaceuticals, they include vaccines, whole blood, blood components, allergenics, somatic cells, gene therapies, tissues, recombinant therapeutic protein, and living medicines used in cell therapy. Biologics can be composed of sugars, proteins, nucleic acids, or complex combinations of these substances, or may be living cells or tissues. They are isolated from living sources—human, animal, plant, fungal, or microbial. They can be used in both human and animal medicine.
Eye banks recover, prepare and deliver donated eyes for cornea transplants and research. The first successful cornea transplant was performed in 1905 and the first eye bank was founded in 1944. Currently, in the United States, eye banks provide tissue for over 80,000 cornea transplants each year to treat conditions such as keratoconus and corneal scarring. In some cases, the white of the eye (sclera) is used to surgically repair recipient eyes. Unlike other organs and tissues, there is an adequate supply of corneas for transplants in the United States, and excess tissue is exported internationally, where there are shortages in many countries, due to greater demand and a less-developed eye banking infrastructure.

Organ printing utilizes techniques similar to conventional 3D printing where a computer model is fed into a printer that lays down successive layers of plastics or wax until a 3D object is produced. In the case of organ printing, the material being used by the printer is a biocompatible plastic. The biocompatible plastic forms a scaffold that acts as the skeleton for the organ that is being printed. As the plastic is being laid down, it is also seeded with human cells from the patient's organ that is being printed for. After printing, the organ is transferred to an incubation chamber to give the cells time to grow. After a sufficient amount of time, the organ is implanted into the patient.

Fecal microbiota transplant (FMT), also known as a stool transplant, is the process of transferring fecal bacteria and other microbes from a healthy individual into another individual. FMT is an effective treatment for Clostridioides difficile infection (CDI). For recurrent CDI, FMT is more effective than vancomycin.
The Center for Biologics Evaluation and Research (CBER) is one of six main centers for the U.S. Food and Drug Administration (FDA), which is a part of the U.S. Department of Health and Human Services. The current Director of CBER is Peter Marks, M.D., PhD. CBER is responsible for assuring the safety, purity, potency, and effectiveness of biologics and related products. Not all biologics are regulated by CBER. Monoclonal antibodies and other therapeutic proteins are regulated by the FDA Center for Drug Evaluation and Research (CDER).

A biobank is a type of biorepository that stores biological samples for use in research. Biobanks have become an important resource in medical research, supporting many types of contemporary research like genomics and personalized medicine.
Good tissue practice (GTP) is one of the "GxP" requirements derived from good manufacturing practice. The rule was written and is enforced by the U.S. Food and Drug Administration (FDA), specifically the Center for Biologics Evaluation and Research. The authority for the regulation comes from the Public Health Service Act and all of the requirements relate to transmission of communicable disease, including bacterial or fungal contamination during processing.
A biorepository is a facility that collects, catalogs, and stores samples of biological material for laboratory research. Biorepositories collect and manage specimens from animals, plants, and other living organisms. Biorepositories store many different types of specimens, including samples of blood, urine, tissue, cells, DNA, RNA, and proteins. If the samples are from people, they may be stored with medical information along with written consent to use the samples in laboratory studies.
Transplantable organs and tissues may both refer to organs and tissues that are relatively often or routinely transplanted, as well as relatively seldom transplanted organs and tissues and ones on the experimental stage.
A Tumor Bank, A Tumor Bank is sometimes also referred to as a Tissue Bank, since normal tissues for research are also often collected. However, this function is distinct from a Tissue Bank which collects and harvests human cadaver tissue for medical research and education, and banks which store Biomedical tissue for organ transplantation.
United States of America v. Regenerative Sciences, LLC, 741 F.3d 1314, was a decision in the United States Court of Appeals for the District of Columbia Circuit filed on February 4, 2014 concerning more than minimally manipulated cell therapies and whether they are considered part of medical practice or a drug, the latter subjecting it to regulation under the Food and Drug Administration (FDA). Regenerative Sciences LLC marketed a therapy procedure called Regenexx-C for the treatment of arthritis and orthopedic injury that involved extraction and culture of mesenchymal stem cells from the same patient which were later reinjected. In 2008, The FDA notified Regenerative Sciences LLC that the procedure may not be in compliance with their regulation using an Untitled Letter, which began a series of suits and counter suits, leading to the 2014 decision upholding the FDA’s regulation of more than minimally manipulated stem cell therapies.
Medical device design, as the name suggests, refers to the design of medical devices. Due to the large amount of regulations in the industry, the design of medical devices presents significant challenges from both engineering and legal perspectives.