1,2-Bis(diphenylphosphino)ethane (dppe) is an organophosphorus compound with the formula (Ph2PCH2)2 (Ph = phenyl). It is a commonly used bidentate ligand in coordination chemistry. It is a white solid that is soluble in organic solvents.

Molybdenum(V) chloride is the inorganic compound with the empirical formula MoCl5. This dark volatile solid is used in research to prepare other molybdenum compounds. It is moisture-sensitive and soluble in chlorinated solvents.
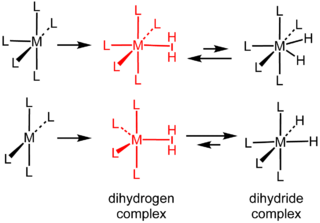
Dihydrogen complexes are coordination complexes containing intact H2 as a ligand. They are a subset of sigma complexes. The prototypical complex is W(CO)3(PCy3)2(H2). This class of compounds represent intermediates in metal-catalyzed reactions involving hydrogen. Hundreds of dihydrogen complexes have been reported. Most examples are cationic transition metals complexes with octahedral geometry.

1,1′-Bis(diphenylphosphino)ferrocene, commonly abbreviated dppf, is an organophosphorus compound commonly used as a ligand in homogeneous catalysis. It contains a ferrocene moiety in its backbone, and is related to other bridged diphosphines such as 1,2-bis(diphenylphosphino)ethane (dppe).

Transition metal dinitrogen complexes are coordination compounds that contain transition metals as ion centers the dinitrogen molecules (N2) as ligands.

Diphosphines, sometimes called bisphosphanes, are organophosphorus compounds most commonly used as bidentate phosphine ligands in inorganic and organometallic chemistry. They are identified by the presence of two phosphino groups linked by a backbone, and are usually chelating. A wide variety of diphosphines have been synthesized with different linkers and R-groups. Alteration of the linker and R-groups alters the electronic and steric properties of the ligands which can result in different coordination geometries and catalytic behavior in homogeneous catalysts.

1,1-Bis(diphenylphosphino)methane (dppm), is an organophosphorus compound with the formula CH2(PPh2)2. Dppm, a white, crystalline powder, is used in inorganic and organometallic chemistry as a ligand. It is more specifically a chelating ligand because it is a ligand that can bond to metals with two phosphorus donor atoms. The natural bite angle is 73°.

Molybdenum tetrachloride is the inorganic compound with the empirical formula MoCl4. The material exists as two polymorphs, both being dark-colored paramagnetic solids. These compounds are mainly of interest as precursors to other molybdenum complexes.

1,2-Bis(dimethylphosphino)ethane (dmpe) is a diphosphine ligand in coordination chemistry. It is a colorless, air-sensitive liquid that is soluble in organic solvents. With the formula (CH2PMe2)2, dmpe is used as a compact strongly basic spectator ligand (Me = methyl), Representative complexes include V(dmpe)2(BH4)2, Mn(dmpe)2(AlH4)2, Tc(dmpe)2(CO)2Cl, and Ni(dmpe)Cl2.
Metal nitrido complexes are coordination compounds and metal clusters that contain an atom of nitrogen bound only to transition metals. These compounds are molecular, i.e. discrete in contrast to the polymeric, dense nitride materials that are useful in materials science. The distinction between the molecular and solid-state polymers is not always very clear as illustrated by the materials Li6MoN4 and more condensed derivatives such as Na3MoN3. Transition metal nitrido complexes have attracted interest in part because it is assumed that nitrogen fixation proceeds via nitrido intermediates. Nitrido complexes have long been known, the first example being salts of [OsO3N]−, described in the 19th century.

A metal-phosphine complex is a coordination complex containing one or more phosphine ligands. Almost always, the phosphine is an organophosphine of the type R3P (R = alkyl, aryl). Metal phosphine complexes are useful in homogeneous catalysis. Prominent examples of metal phosphine complexes include Wilkinson's catalyst (Rh(PPh3)3Cl), Grubbs' catalyst, and tetrakis(triphenylphosphine)palladium(0).

Molybdenum(III) chloride is the inorganic compound with the formula MoCl3. It forms purple crystals.

Chlorobis(dppe)iron hydride is a coordination complex with the formula HFeCl(dppe)2, where dppe is the bidentate ligand 1,2-bis(diphenylphosphino)ethane. It is a red-violet solid. The compound has attracted much attention as a precursor to dihydrogen complexes.

Tris(trimethylsilyl)amine is the simplest tris(trialkylsilyl)amine which are having the general formula (R3Si)3N, in which all three hydrogen atoms of the ammonia are replaced by trimethylsilyl groups (-Si(CH3)3). Tris(trimethylsilyl)amine has been for years in the center of scientific interest as a stable intermediate in chemical nitrogen fixation (i. e. the conversion of atmospheric nitrogen N2 into organic substrates under normal conditions).

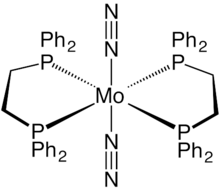
Transition metal nitrile complexes are coordination compounds containing nitrile ligands. Because nitriles are weakly basic, the nitrile ligands in these complexes are often labile.
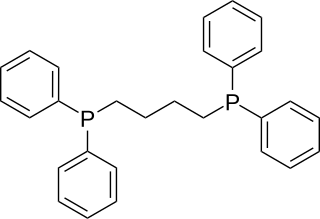
1,4-Bis(diphenylphosphino)butane (dppb) is an organophosphorus compound with the formula (Ph2PCH2CH2)2. It is less commonly used in coordination chemistry than other diphosphine ligands such as dppe. It is a white solid that is soluble in organic solvents.

Dichloro[1,2-bis(diphenylphosphino)ethane]nickel is a coordination complex with the formula NiCl2(dppe); where dppe is the diphosphine 1,2-bis(diphenylphosphino)ethane. It is used as a reagent and as a catalyst. The compound is a bright orange-red diamagnetic solid. The complex adopts a square planar geometry.

Hexa(tert-butoxy)dimolybdenum(III) is a coordination complex of molybdenum(III). It is one of the homoleptic alkoxides of molybdenum. An orange, air-sensitive solid, the complex has attracted academic attention as the precursor to many organomolybdenum derivatives. It an example of a charge-neutral complex featuring a molybdenum to molybdenum triple bond (Mo≡Mo), arising from the coupling of a pair of d3 metal centers. It can be prepared by a salt metathesis reaction from the THF complex of molybdenum trichloride and lithium tert-butoxide:
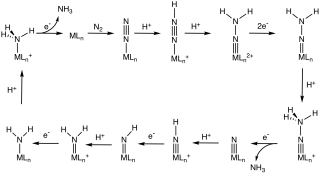
Abiological nitrogen fixation describes chemical processes that fix (react with) N2, usually with the goal of generating ammonia. The dominant technology for abiological nitrogen fixation is the Haber process, which uses an iron-based heterogeneous catalysts and H2 to convert N2 to NH3. This article focuses on homogeneous (soluble) catalysts for the same or similar conversions.

In chemistry, a transition metal ether complex is a coordination complex consisting of a transition metal bonded to one or more ether ligand. The inventory of complexes is extensive. Common ether ligands are diethyl ether and tetrahydrofuran. Common chelating ether ligands include the glymes, dimethoxyethane (dme) and diglyme, and the crown ethers. Being lipophilic, metal-ether complexes often exhibit solubility in organic solvents, a property of interest in synthetic chemistry. In contrast, the di-ether 1,4-dioxane is generally a bridging ligand.