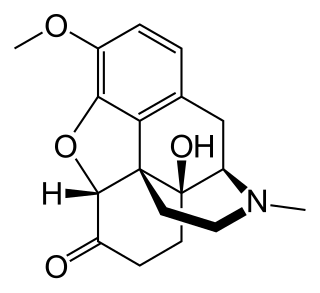
Oxycodone, sold under the brand names Roxicodone and OxyContin among others, is an opioid medication used for treatment of moderate to severe pain. It is highly addictive and a common drug of abuse. It is usually taken by mouth, and is available in immediate-release and controlled-release formulations. Onset of pain relief typically begins within fifteen minutes and lasts for up to six hours with the immediate-release formulation. In the United Kingdom, it is available by injection. Combination products are also available with paracetamol (acetaminophen), ibuprofen, naloxone, naltrexone, and aspirin.

Dextromethorphan, often referred to as DXM, is a medication most often used as a cough suppressant in over-the-counter cold and cough medicines. It is sold in syrup, tablet, spray, and lozenge forms.

Ketobemidone, sold under the brand name Ketogan among others, is a powerful synthetic opioid painkiller. Its effectiveness against pain is in the same range as morphine, and it also has some NMDA-antagonist properties imparted, in part, by its metabolite norketobemidone. This may make it useful for some types of pain that do not respond well to other opioids. It is marketed in Denmark, Iceland, Norway and Sweden and is used for severe pain.

Nicocodeine is an opioid analgesic and cough suppressant, an ester of codeine closely related to dihydrocodeine and the codeine analogue of nicomorphine. It is not commonly used in most countries, but has activity similar to other opiates. Nicocodeine and nicomorphine were introduced in 1957 by Lannacher Heilmittel of Austria. Nicocodeine is metabolised in the liver by demethylation to produce nicomorphine, also known as 6-nicotinoylmorphine, and subsequently further metabolised to morphine. Side effects are similar to those of other opiates and include itching, nausea and respiratory depression. Related opioid analogues such as nicomorphine and nicodicodeine were first synthesized. The definitive synthesis, which involves treating anhydrous codeine base with nicotinic anhydride at 130 °C, was published by Pongratz and Zirm in Monatshefte für Chemie in 1957, simultaneously with the two analogues in an article about amides and esters of various organic acids.

Nicodicodine is an opioid developed as a cough suppressant and analgesic. Synthesized in 1904, it is not commonly used, but has activity similar to other opioids. Nicodicodine is metabolised in the liver by demethylation to produce 6-nicotinoyldihydromorphine, and subsequently further metabolised to dihydromorphine. Since the final active metabolite is the slightly stronger opiate dihydromorphine rather than morphine, nicodicodine can be expected to be marginally more potent and longer acting than nicocodeine. Side effects are similar to those of other opioids and include itching, nausea and respiratory depression.

Alvimopan is a drug which behaves as a peripherally acting μ-opioid receptor antagonist. With the limited ability to cross the blood–brain barrier and reach the μ-opioid receptors of the central nervous system, the clinically undesirable effects of centrally acting opioid antagonists are avoided without affecting the intended blockade of μ-opioid receptors in the gastrointestinal tract. It is currently only Food and Drug Administration approved for the treatment of postoperative ileus which it received in May 2008.
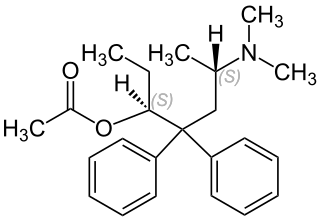
Levacetylmethadol (INN), levomethadyl acetate (USAN), OrLAAM or levo-α-acetylmethadol (LAAM) is a synthetic opioid similar in structure to methadone. It has a long duration of action due to its active metabolites.

Nalorphine (INN), also known as N-allylnormorphine, is a mixed opioid agonist–antagonist with opioid antagonist and analgesic properties. It was introduced in 1954 and was used as an antidote to reverse opioid overdose and in a challenge test to determine opioid dependence.

Oripavine is an opioid and the major metabolite of thebaine. It is the parent compound from which a series of semi-synthetic opioids are derived, which includes the compounds etorphine and buprenorphine. Although its analgesic potency is comparable to morphine, it is not used clinically due to its severe toxicity and low therapeutic index. Due to its use in manufacture of strong opioids, oripavine is a controlled substance in some jurisdictions.
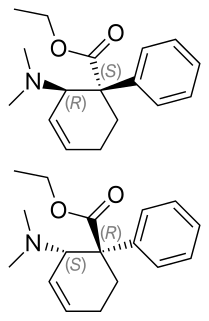
Tilidine, or tilidate is a synthetic opioid painkiller, used mainly in Belgium, Bulgaria, Germany, Luxembourg, South Africa and Switzerland for the treatment of moderate to severe pain, both acute and chronic. Its onset of pain relief after oral administration is about 10–15 minutes and peak relief from pain occurs about 25–50 minutes after oral administration.

Normorphine is an opiate analogue, the N-demethylated derivative of morphine, that was first described in the 1950s when a large group of N-substituted morphine analogues were characterized for activity. The compound has relatively little opioid activity in its own right, but is a useful intermediate which can be used to produce both opioid antagonists such as nalorphine, and also potent opioid agonists such as N-phenethylnormorphine. with its formation from morphine catalyzed by the liver enzymes CYP3A4 and CYP2C8.

RWJ-394674 is a drug that is used in scientific research. It is a potent, orally active analgesic drug that produces little respiratory depression. RWJ-394674 itself is a potent and selective agonist for δ-opioid receptors, with a Ki of 0.24 nM at δ and 72 nM at μ. However once inside the body, RWJ-394674 is dealkylated to its monodesethyl metabolite RWJ-413216, which is a potent agonist at the μ-opioid receptor and has less affinity for δ. The effect of RWJ-394674 when administered in vivo thus produces potent agonist effects at both μ and δ receptors through the combined actions of the parent drug and its active metabolite, with the δ-agonist effects counteracting the respiratory depression from the μ-opioid effects, and the only prominent side-effect being sedation.

Norpropoxyphene is a major metabolite of the opioid analgesic drug dextropropoxyphene, and is responsible for many of the side effects associated with use of this drug, especially the unusual toxicity seen during dextropropoxyphene overdose. It has weaker analgesic effects than dextropropoxyphene itself, but is a relatively potent pro-convulsant and blocker of sodium and potassium channels, particularly in heart tissue, which produces prolonged intracardiac conduction time and can lead to heart failure following even relatively minor overdoses. The toxicity of this metabolite makes dextropropoxyphene up to 10 times more likely to cause death following overdose compared to other similar mild opioid analgesics, and has led to dextropropoxyphene being withdrawn from the market in some countries.

An opiate, in classical pharmacology, is a substance derived from opium. In more modern usage, the term opioid is used to designate all substances, both natural and synthetic, that bind to opioid receptors in the brain. Opiates are alkaloid compounds naturally found in the opium poppy plant Papaver somniferum. The psychoactive compounds found in the opium plant include morphine, codeine, and thebaine. Opiates have long been used for a variety of medical conditions with evidence of opiate trade and use for pain relief as early as the eighth century AD. Opiates are considered drugs with moderate to high abuse potential and are listed on various "Substance-Control Schedules" under the Uniform Controlled Substances Act of the United States of America.

Nortilidine is the major active metabolite of tilidine. It is formed from tilidine by demethylation in the liver. The racemate has opioid analgesic effects roughly equivalent in potency to that of morphine. The (1R,2S) isomer has NMDA antagonist activity. The drug also acts as a dopamine reuptake inhibitor. The reversed-ester of nortilidine is also known, as is the corresponding analogue with the cyclohexene ring replaced by cyclopentane, which have almost identical properties to nortilidine.
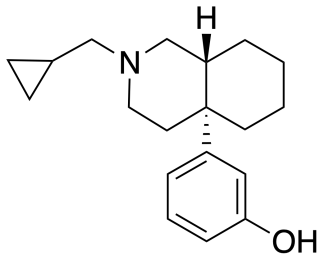
Ciprefadol is an opioid analgesic that is an isoquinoline derivative most closely related to cyclazocine and picenadol, with a number of other related compounds known. Ciprefadol is a mixed agonist–antagonist at μ-opioid receptors and can partly block the effects of morphine at low doses, though at higher doses it acts more like a full agonist. It is also a potent κ-opioid agonist, unlike the corresponding N-methyl and N-phenethyl derivatives which are reasonably μ-selective agonists.

Benzhydrocodone (INN) is an opioid prodrug of the morphinan class. Its chemical structure consists of hydrocodone coupled with benzoic acid. Benzhydrocodone itself is inactive and acts as a prodrug to hydrocodone upon cleavage of the benzoate portion of the molecule.
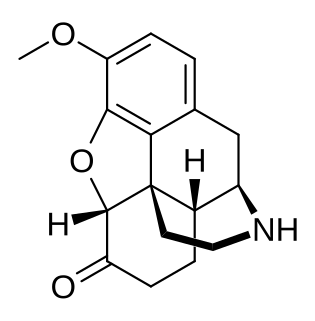
Norhydrocodone is the major metabolite of the opioid analgesic hydrocodone. It is formed from hydrocodone in the liver via N-demethylation predominantly by CYP3A4. Unlike hydromorphone, a minor metabolite of hydrocodone, norhydrocodone is described as inactive. However, norhydrocodone is actually an agonist of the μ-opioid receptor with similar potency to hydrocodone, but has been found to produce only minimal analgesia when administered peripherally to animals. This is likely due to poor blood-brain-barrier and thus central nervous system penetration.
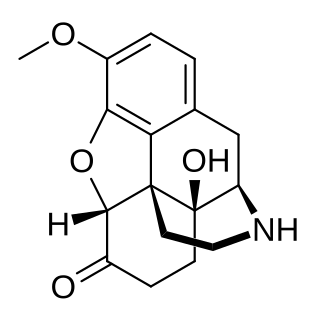
Noroxycodone is the major metabolite of the opioid analgesic oxycodone. It is formed from oxycodone in the liver via N-demethylation predominantly by CYP3A4. Noroxycodone binds to and activates the μ-opioid receptor (MOR) similarly to oxycodone, although with one-third of the affinity of oxycodone and 5- to 10-fold lower activational potency. However, although a potent MOR agonist, noroxycodone poorly crosses the blood-brain-barrier into the central nervous system, and for this reason, is only minimally analgesic in comparison.

Desmethylchlorotrianisene (DMCTA) is a nonsteroidal estrogen which is thought to be the major active metabolite of chlorotrianisene. It is a 1:1 mixture of cis and trans isomers. DMCTA is produced from CTA via mono-O-demethylation catalyzed by cytochrome P450 enzymes in the liver. CTA is thought to act as a long-lasting prodrug of DMCTA.




















