
A plant canker is a small area of dead tissue, which grows slowly, often over years. Some cankers are of only minor consequence, but others are ultimately lethal and therefore can have major economic implications for agriculture and horticulture. Their causes include a wide range of organisms as fungi, bacteria, mycoplasmas and viruses. The majority of canker-causing organisms are bound to a unique host species or genus, but a few will attack other plants. Weather and animals can spread canker, thereby endangering areas that have only slight amount of canker.
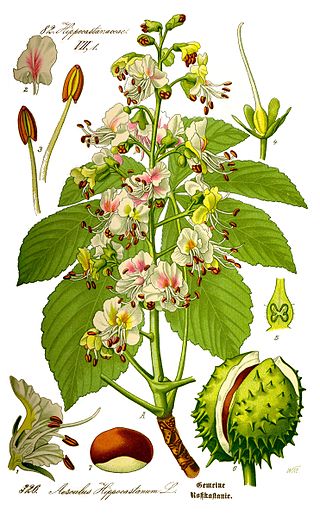
Aesculus hippocastanum, the horse chestnut, is a species of flowering plant in the maple, soapberry and lychee family Sapindaceae. It is a large, deciduous, synoecious (hermaphroditic-flowered) tree. It is also called horse-chestnut, European horsechestnut, buckeye, and conker tree. It is not to be confused with the Spanish chestnut, Castanea sativa, which is a tree in another family, Fagaceae.

A leaf spot is a limited, discoloured, diseased area of a leaf that is caused by fungal, bacterial or viral plant diseases, or by injuries from nematodes, insects, environmental factors, toxicity or herbicides. These discoloured spots or lesions often have a centre of necrosis. Symptoms can overlap across causal agents, however differing signs and symptoms of certain pathogens can lead to the diagnosis of the type of leaf spot disease. Prolonged wet and humid conditions promote leaf spot disease and most pathogens are spread by wind, splashing rain or irrigation that carry the disease to other leaves.

Citrus canker is a disease affecting Citrus species caused by the bacterium Xanthomonas. Infection causes lesions on the leaves, stems, and fruit of citrus trees, including lime, oranges, and grapefruit. While not harmful to humans, canker significantly affects the vitality of citrus trees, causing leaves and fruit to drop prematurely; a fruit infected with canker is safe to eat, but too unsightly to be sold. Citrus canker is mainly a leaf-spotting and rind-blemishing disease, but when conditions are highly favorable, it can cause defoliation, shoot dieback, and fruit drop.

Pseudomonas syringae is a rod-shaped, Gram-negative bacterium with polar flagella. As a plant pathogen, it can infect a wide range of species, and exists as over 50 different pathovars, all of which are available to researchers from international culture collections such as the NCPPB, ICMP, and others.
Pseudomonas avellanae is a Gram-negative plant pathogenic bacterium. It is the causal agent of bacterial canker of hazelnut. Based on 16S rRNA analysis, P. avellanae has been placed in the P. syringae group. This species was once included as a pathovar of Pseudomonas syringae, but following DNA-DNA hybridization, it was instated as a separate species. Following ribotypical analysis Pseudomonas syringae pv. theae was incorporated into this species.

Pseudomonas cichorii is a Gram-negative soil bacterium that is pathogenic to plants. It has a wide host range, and can have an important economical impact on lettuce, celery and chrysanthemum crops. P. cichorii was first isolated on endives, from which it derives its name. It produces 6-aminopenicillanic acid. Based on 16S rRNA analysis, P. cichorii has been placed in the P. syringae group.

Pseudomonas savastanoi is a gram-negative plant pathogenic bacterium that infects a variety of plants. It was once considered a pathovar of Pseudomonas syringae, but following DNA-relatedness studies, it was instated as a new species. It is named after Savastano, a worker who proved between 1887 and 1898 that olive knot are caused by bacteria.
Pseudomonas viridiflava is a fluorescent, Gram-negative, soil bacterium that is pathogenic to plants. It was originally isolated from the dwarf or runner bean, in Switzerland. Based on 16S rRNA analysis, P. viridiflava has been placed in the P. syringae group. Following ribotypical analysis misidentified strains of Pseudomonas syringae pv. ribicola and Pseudomonas syringae pv. primulae were incorporated into this species. This pathogen causes bacterial blight of Kiwifruit.
Pseudomonas amygdali is a Gram-negative plant pathogenic bacterium. It is named after its ability to cause disease on almond trees. Different analyses, including 16S rRNA analysis, DNA-DNA hybridization, and MLST clearly placed P. amygdali in the P. syringae group together with the species Pseudomonas ficuserectae and Pseudomonas meliae, and 27 pathovars of Pseudomonas syringae/Pseudomonas savastanoi, constituting a single, well-defined phylogenetic group which should be considered as a single species. This phylogenetic group has not been formally named because of the lack of reliable means to differentiate it phenotypically from closely related species, and it is currently known as either genomospecies 2 or phylogroup 3. When it is formally named, the correct name for this new species should be Pseudomonas amygdali, which takes precedence over all the other names of taxa from this group, including Pseudomonas savastanoi, which is and inadequate and confusing name whose use is not recommended.

Pseudomonas cannabina is a gray, Gram-negative, fluorescent, motile, flagellated, aerobic bacterium that causes leaf and stem rot of hemp, from which it derives its name. It was formerly classified as a pathovar of Pseudomonas syringae, but following ribotypical analysis, it was reinstated as a species. The type strain is CFBP 2341.

Halo blight of bean is a bacterial disease caused by Pseudomonas syringae pv. phaseolicola. Halo blight’s pathogen is a gram-negative, aerobic, polar-flagellated and non-spore forming bacteria. This bacterial disease was first discovered in the early 1920s, and rapidly became the major disease of beans throughout the world. The disease favors the places where temperatures are moderate and plentiful inoculum is available.

Clavibacter michiganensis is an aerobic non-sporulating Gram-positive plant pathogenic actinomycete of the genus Clavibacter. Clavibacter michiganensis has several subspecies. Clavibacter michiganensis subsp. michiganensis causes substantial economic losses worldwide by damaging tomatoes and potatoes.

"Pseudomonas tomato" is a Gram-negative plant pathogenic bacterium that infects a variety of plants. It was once considered a pathovar of Pseudomonas syringae, but following DNA-relatedness studies, it was recognized as a separate species and several other former P. syringae pathovars were incorporated into it. Since no official name has yet been given, it is referred to by the epithet 'Pseudomonas tomato' .

Phytophthora kernoviae is a plant pathogen that mainly infects European beech and Rhododendron ponticum. It was first identified in 2003 in Cornwall, UK when scientists were surveying for the presence of Phytophthora ramorum. This made it the third new Phytophthora species to be found in the UK in a decade. It was named Phytophthora kernoviae after the ancient name for Cornwall, Kernow. It causes large stem lesions on beech and necrosis of stems and leaves of Rhododendron ponticum. It is self-fertile. It has also been isolated from Quercus robur and Liriodendron tulipifera. The original paper describing the species, stated it can infect Magnolia and Camellia species, Pieris formosa, Gevuina avellana, Michelia doltsopa and Quercus ilex. Since then many other plants have been identified as natural hosts of the pathogen. Molecular analysis has revealed that an infection on Pinus radiata, recorded in New Zealand in 1950, was caused by P. kernoviae. The pathogen was also noted on Drimys winteri, Gevuina avellana, Ilex aquifolium, Quercus ilex, Vaccinium myrtillus, Hedera helix, Podocarpus salignas.
Black rot, caused by the bacterium Xanthomonas campestris pv. campestris (Xcc), is considered the most important and most destructive disease of crucifers, infecting all cultivated varieties of brassicas worldwide. This disease was first described by botanist and entomologist Harrison Garman in Lexington, Kentucky, US in 1889. Since then, it has been found in nearly every country in which vegetable brassicas are commercially cultivated.

Xanthomonas campestris pv. vesicatoria is a bacterium that causes bacterial leaf spot (BLS) on peppers and tomatoes. It is a gram-negative and rod-shaped. It causes symptoms throughout the above-ground portion of the plant including leaf spots, fruit spots and stem cankers. Since this bacterium cannot live in soil for more than a few weeks and survives as inoculum on plant debris, removal of dead plant material and chemical applications to living plants are considered effective control mechanisms.
Black pod disease is a protozoal disease of Cocoa trees. This pathogen if left untreated can destroy all yields; annually the pathogen can cause a yield loss of up to 1/3 and up to 10% of total trees can be lost completely.

Bacterial blight of soybean is a widespread disease of soybeans caused by Pseudomonas syringaepv. glycinea.
Xanthomonas campestris pv. juglandis is an anaerobic, Gram negative, rod-shaped bacteria that can affect walnut trees though the flowers, buds, shoots, branches, trunk, and fruit. It can have devastating effects including premature fruit drop and lesions on the plant. This pathogen was first isolated by Newton B. Pierce in California in 1896 and was then named Pseudomonas juglandis. In 1905 it was reclassified as Bacterium juglandis, in 1930 it became Phytomas juglandis, and in 1939 it was named Xanthomas juglandis. The International Standards for Naming Pathovars declared it to be named Xanthomonas campestris pv. juglandis in 1980. There have been recent proposals to change the name once again to Xanthomonas arboricola pv. juglandis, but this has not yet been universally accepted.














