A bone is a rigid organ that constitutes part of the skeleton in most vertebrate animals. Bones protect the various other organs of the body, produce red and white blood cells, store minerals, provide structure and support for the body, and enable mobility. Bones come in a variety of shapes and sizes and have complex internal and external structures. They are lightweight yet strong and hard and serve multiple functions.
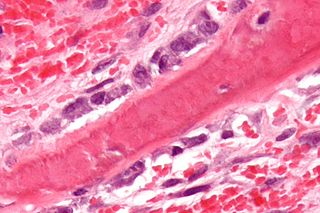
Osteoblasts are cells with a single nucleus that synthesize bone. However, in the process of bone formation, osteoblasts function in groups of connected cells. Individual cells cannot make bone. A group of organized osteoblasts together with the bone made by a unit of cells is usually called the osteon.

The periosteum is a membrane that covers the outer surface of all bones, except at the articular surfaces of long bones. Endosteum lines the inner surface of the medullary cavity of all long bones.

An osteocyte, an oblate shaped type of bone cell with dendritic processes, is the most commonly found cell in mature bone. It can live as long as the organism itself. The adult human body has about 42 billion of them. Osteocytes do not divide and have an average half life of 25 years. They are derived from osteoprogenitor cells, some of which differentiate into active osteoblasts. Osteoblasts/osteocytes develop in mesenchyme.

Chondrocytes are the only cells found in healthy cartilage. They produce and maintain the cartilaginous matrix, which consists mainly of collagen and proteoglycans. Although the word chondroblast is commonly used to describe an immature chondrocyte, the term is imprecise, since the progenitor of chondrocytes can differentiate into various cell types, including osteoblasts.
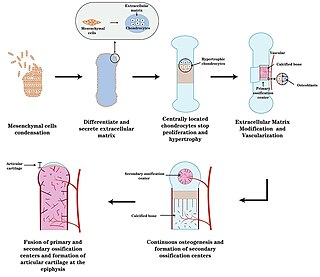
Endochondral ossification is one of the two essential pathways by which bone tissue is produced during fetal development of the mammalian skeletal system, the other pathway being intramembranous ossification. Both endochondral and intramembranous processes initiate from a precursor mesenchymal tissue, but their transformations into bone are different. In intramembranous ossification, mesenchymal tissue is directly converted into bone. On the other hand, endochondral ossification starts with mesenchymal tissue turning into an intermediate cartilage stage, which is eventually substituted by bone.

Intramembranous ossification is one of the two essential processes during fetal development of the gnathostome skeletal system by which rudimentary bone tissue is created. Intramembranous ossification is also an essential process during the natural healing of bone fractures and the rudimentary formation of bones of the head.
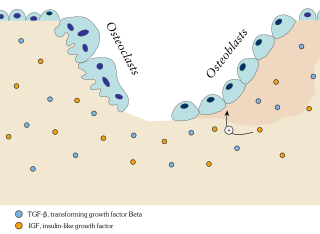
Ossification in bone remodeling is the process of laying down new bone material by cells named osteoblasts. It is synonymous with bone tissue formation. There are two processes resulting in the formation of normal, healthy bone tissue: Intramembranous ossification is the direct laying down of bone into the primitive connective tissue (mesenchyme), while endochondral ossification involves cartilage as a precursor.

Bone resorption is resorption of bone tissue, that is, the process by which osteoclasts break down the tissue in bones and release the minerals, resulting in a transfer of calcium from bone tissue to the blood.

Chondrogenesis is the biological process through which cartilage tissue is formed and developed. This intricate and tightly regulated cellular differentiation pathway plays a crucial role in skeletal development, as cartilage serves as a fundamental component of the embryonic skeleton. The term "chondrogenesis" is derived from the Greek words "chondros," meaning cartilage, and "genesis," meaning origin or formation.

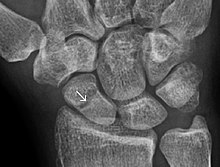
Short bones are designated as those bones that are more or less equal in length, width, and thickness. They include the tarsals in the ankle and the carpals in the wrist. They are one of five types of bones: short, long, flat, irregular and sesamoid. Most short bones are named according to their shape as they exhibit a variety of complex morphological features

The brown tumor is a bone lesion that arises in settings of excess osteoclast activity, such as hyperparathyroidism. They are a form of osteitis fibrosa cystica. It is not a neoplasm, but rather simply a mass. It most commonly affects the maxilla and mandible, though any bone may be affected. Brown tumours are radiolucent on x-ray.

In osteology, bone remodeling or bone metabolism is a lifelong process where mature bone tissue is removed from the skeleton and new bone tissue is formed. These processes also control the reshaping or replacement of bone following injuries like fractures but also micro-damage, which occurs during normal activity. Remodeling responds also to functional demands of the mechanical loading.

Transcription factor Sp7, also called Osterix (Osx), is a protein that in humans is encoded by the SP7 gene. It is a member of the Sp family of zinc-finger transcription factors It is highly conserved among bone-forming vertebrate species It plays a major role, along with Runx2 and Dlx5 in driving the differentiation of mesenchymal precursor cells into osteoblasts and eventually osteocytes. Sp7 also plays a regulatory role by inhibiting chondrocyte differentiation maintaining the balance between differentiation of mesenchymal precursor cells into ossified bone or cartilage. Mutations of this gene have been associated with multiple dysfunctional bone phenotypes in vertebrates. During development, a mouse embryo model with Sp7 expression knocked out had no formation of bone tissue. Through the use of GWAS studies, the Sp7 locus in humans has been strongly associated with bone mass density. In addition there is significant genetic evidence for its role in diseases such as Osteogenesis imperfecta (OI).

Osteochondroprogenitor cells are progenitor cells that arise from mesenchymal stem cells (MSC) in the bone marrow. They have the ability to differentiate into osteoblasts or chondrocytes depending on the signalling molecules they are exposed to, giving rise to either bone or cartilage respectively. Osteochondroprogenitor cells are important for bone formation and maintenance.
The in vivo bioreactor is a tissue engineering paradigm that uses bioreactor methodology to grow neotissue in vivo that augments or replaces malfunctioning native tissue. Tissue engineering principles are used to construct a confined, artificial bioreactor space in vivo that hosts a tissue scaffold and key biomolecules necessary for neotissue growth. Said space often requires inoculation with pluripotent or specific stem cells to encourage initial growth, and access to a blood source. A blood source allows for recruitment of stem cells from the body alongside nutrient delivery for continual growth. This delivery of cells and nutrients to the bioreactor eventually results in the formation of a neotissue product.

Orthopedic surgery is the branch of surgery concerned with conditions involving the musculoskeletal system. Orthopedic surgeons use both surgical and nonsurgical means to treat musculoskeletal injuries, sports injuries, degenerative diseases, infections, bone tumours, and congenital limb deformities. Trauma surgery and traumatology is a sub-specialty dealing with the operative management of fractures, major trauma and the multiply-injured patient.
A bone growth factor is a growth factor that stimulates the growth of bone tissue.
Craniofacial regeneration refers to the biological process by which the skull and face regrow to heal an injury. This page covers birth defects and injuries related to the craniofacial region, the mechanisms behind the regeneration, the medical application of these processes, and the scientific research conducted on this specific regeneration. This regeneration is not to be confused with tooth regeneration. Craniofacial regrowth is broadly related to the mechanisms of general bone healing.
Joints form during embryonic development in conjunction with the formation and growth of the associated bones. The joints and bones are developed from the embryonic tissue called mesenchyme.