Related Research Articles

An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting bacterial infections, and antibiotic medications are widely used in the treatment and prevention of such infections. They may either kill or inhibit the growth of bacteria. A limited number of antibiotics also possess antiprotozoal activity. Antibiotics are not effective against viruses such as the common cold or influenza; drugs which inhibit viruses are termed antiviral drugs or antivirals rather than antibiotics.
A superinfection is a second infection superimposed on an earlier one, especially by a different microbial agent of exogenous or endogenous origin, that is resistant to the treatment being used against the first infection. Examples of this in bacteriology are the overgrowth of endogenous Clostridium difficile that occurs following treatment with a broad-spectrum antibiotic, and pneumonia or sepsis from Pseudomonas aeruginosa in some immunocompromised patients.
The National Cancer Institute (NCI) coordinates the United States National Cancer Program and is part of the National Institutes of Health (NIH), which is one of eleven agencies that are part of the U.S. Department of Health and Human Services. The NCI conducts and supports research, training, health information dissemination, and other activities related to the causes, prevention, diagnosis, and treatment of cancer; the supportive care of cancer patients and their families; and cancer survivorship.
An antimicrobial is an agent that kills microorganisms or stops their growth. Antimicrobial medicines can be grouped according to the microorganisms they act primarily against. For example, antibiotics are used against bacteria, and antifungals are used against fungi. They can also be classified according to their function. Agents that kill microbes are microbicides, while those that merely inhibit their growth are called bacteriostatic agents. The use of antimicrobial medicines to treat infection is known as antimicrobial chemotherapy, while the use of antimicrobial medicines to prevent infection is known as antimicrobial prophylaxis.

Pralatrexate, sold under the brand name Folotyn, is a medication used for the treatment of relapsed or refractory peripheral T-cell lymphoma (PTCL).

Piperacillin is a broad-spectrum β-lactam antibiotic of the ureidopenicillin class. The chemical structure of piperacillin and other ureidopenicillins incorporates a polar side chain that enhances penetration into Gram-negative bacteria and reduces susceptibility to cleavage by Gram-negative beta lactamase enzymes. These properties confer activity against the important hospital pathogen Pseudomonas aeruginosa. Thus piperacillin is sometimes referred to as an "anti-pseudomonal penicillin".

Tigecycline, sold under the brand name Tygacil, is an tetracycline antibiotic medication for a number of bacterial infections. It is a glycylcycline administered intravenously. It was developed in response to the growing rate of antibiotic resistant bacteria such as Staphylococcus aureus, Acinetobacter baumannii, and E. coli. As a tetracycline derivative antibiotic, its structural modifications has expanded its therapeutic activity to include Gram-positive and Gram-negative organisms, including those of multi-drug resistance.

Carbapenems are a class of very effective antibiotic agents most commonly used for the treatment of severe bacterial infections. This class of antibiotics is usually reserved for known or suspected multidrug-resistant (MDR) bacterial infections. Similar to penicillins and cephalosporins, carbapenems are members of the beta lactam class of antibiotics, which kill bacteria by binding to penicillin-binding proteins, thus inhibiting bacterial cell wall synthesis. However, these agents individually exhibit a broader spectrum of activity compared to most cephalosporins and penicillins. Furthermore, carbapenems are typically unaffected by emerging antibiotic resistance, even to other beta-lactams.

Imipenem is an intravenous β-lactam antibiotic discovered by Merck scientists Burton Christensen, William Leanza, and Kenneth Wildonger in the mid-1970s. Carbapenems are highly resistant to the β-lactamase enzymes produced by many multiple drug-resistant Gram-negative bacteria, thus play a key role in the treatment of infections not readily treated with other antibiotics.

Rifaximin, is a non-absorbable, a broad spectrum antibiotics mainly used to treat travelers' diarrhea. It is based on the rifamycin antibiotics family. Since its approval in Italy in 1987, it has been licensed in over more than 30 countries for the treatment of a variety of gastrointestinal diseases like irritable bowel syndrome, and hepatic encephalopathy. It acts by inhibiting RNA synthesis in susceptible bacteria by binding to the RNA polymerase enzyme. This binding blocks translocation, which stops transcription. It is marketed under the brand name Xifaxan by Salix Pharmaceuticals.
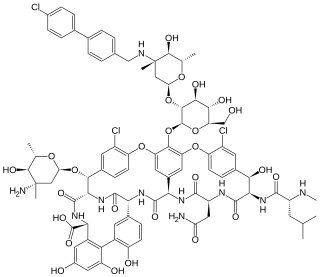
Oritavancin, sold under the brand name Orbactiv among others, is a semisynthetic glycopeptide antibiotic medication for the treatment of serious Gram-positive bacterial infections. Its chemical structure as a lipoglycopeptide is similar to vancomycin.

Cefditoren also known as cefditoren pivoxil is a broad-spectrum antibiotic, taken by mouth to treat pneumonia and other infections. It is a third-generation oral cephalosporin with a broad spectrum of activity against bacterial pathogens, including both Gram-positive and Gram-negative bacteria, and it is effective against some antibiotic-resistant bacteria because it is not susceptible to hydrolysis by many common beta-lactamases. The United States Food and Drug Administration approved cefditoren pivoxil for adults and adolescents in 2001. In 2018 Zuventus Healthcare received approval for cefditoren pivoxil dry powder for suspension for the treatment of mild to moderate infection in children which are caused by susceptible strains of the designated microorganisms.

Beta-lactamases are a family of enzymes involved in bacterial resistance to beta-lactam antibiotics. In bacterial resistance to beta-lactam antibiotics, the bacteria have beta-lactamase which degrade the beta-lactam rings, rendering the antibiotic ineffective. However, with beta-lactamase inhibitors, these enzymes on the bacteria are inhibited, thus allowing the antibiotic to take effect. Strategies for combating this form of resistance have included the development of new beta-lactam antibiotics that are more resistant to cleavage and the development of the class of enzyme inhibitors called beta-lactamase inhibitors. Although β-lactamase inhibitors have little antibiotic activity of their own, they prevent bacterial degradation of beta-lactam antibiotics and thus extend the range of bacteria the drugs are effective against.

Clinafloxacin is an investigational fluoroquinolone antibiotic. Despite its promising antibiotic activity, the clinical development of clinafloxacin has been hampered by its risk for inducing serious side effects.

The Biomedical Advanced Research and Development Authority(BARDA) is a U.S. Department of Health and Human Services (HHS) office responsible for the procurement and development of medical countermeasures, principally against bioterrorism, including chemical, biological, radiological and nuclear (CBRN) threats, as well as pandemic influenza and emerging diseases. BARDA was established in 2006 through the Pandemic and All-Hazards Preparedness Act (PAHPA) and reports to the Office of the Assistant Secretary for Preparedness and Response (ASPR). The office manages Project BioShield, which funds the research, development and stockpiling of vaccines and treatments that the government could use during public health emergencies such as chemical, biological, radiological or nuclear (CBRN) attacks.

The Vaccine Research Center (VRC), is an intramural division of the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH), US Department of Health and Human Services (HHS). The mission of the VRC is to discover and develop both vaccines and antibody-based products that target infectious diseases.

Ceftolozane/tazobactam, sold under the brand name Zerbaxa, is a combination antibiotic medication used for the treatment of complicated urinary tract infections and complicated intra-abdominal infections in adults. Ceftolozane is a cephalosporin antibiotic, developed for the treatment of infections with gram-negative bacteria that are resistant to conventional antibiotics. It was studied for urinary tract infections, intra-abdominal infections and ventilator-associated bacterial pneumonia.
Michael S. Gilmore is an American, focusing in infectious diseases and ocular genomics, currently the Sir William Osler Professor of Ophthalmology (Microbiology), Harvard Medical School, Mike serves as Director of the Infectious Disease Institute, and Co-Director of the Microbial Sciences Initiative of Harvard University. Additionally, he is a Senior Associate Member of the Broad Institute. As Principal Investigator of the Harvard-wide Program on Antibiotic Resistance, his research focuses on the evolution and development of multidrug resistant strains of enterococci, staphylococci, and streptococci, and the development of new therapeutics. He was named by Eric Lander in “The Heroes of CRISPR”3 as inspiring Broad Institute interest in developing CRISPR as a tool for therapeutic gene editing. Mike has trained over 35 graduate students and postdocs, and is currently course coordinator and principle lecturer in the Harvard University course OEB290/MICRO210 Microbiology: Chemistry, ecology and evolution. Outside of Harvard, he serves as chair of the US National Institutes of Health (NIH) blue ribbon panel for the Antimicrobial Resistance Diagnostic Challenge. He is past chair of the NIH Bacterial Pathogenesis Study Section, the Gordon Conference on Microbial Adhesion and Signal Transduction, American Society for Microbiology (ASM) Division D, and the Association for Research in Vision and Ophthalmology (ARVO) IM Section. Mike is founder of the International Conference on Enterococci (ICE) series, and the Boston Area Antibiotic Resistance Network (BAARN). He started his academic career in 1984 at the University of Oklahoma Health Sciences Center, where he rose through the ranks to Vice President for Research. He also held the MG McCool professorship and was awarded the George Lynn Cross research chair. In 2004 he moved to Harvard Medical School as President and CEO of the Schepens Eye Research Institute, Marie and DeWalt Ankeny Director of Research and CL Schepens Professor of Ophthalmology. In 2010, he moved his laboratories to the Massachusetts General Hospital campus, in the Massachusetts Eye and Ear Infirmary. He has published over 200 peer reviewed manuscripts in Cell, Nature, Science, PNAS and other leading journals. He continues to serve on numerous advisory boards and committees for public and private organizations, focused on drug discovery, antibiotic resistance, and bacterial pathogenesis.

Drug repositioning is the repurposing of an approved drug for the treatment of a different disease or medical condition than that for which it was originally developed. This is one line of scientific research which is being pursued to develop safe and effective COVID-19 treatments. Other research directions include the development of a COVID-19 vaccine and convalescent plasma transfusion.
Broad-spectrum antivirals (BSAs) are a class of molecules or compounds, which inhibit the replication of a broad range of viruses. BSAs could be divided into experimental and investigational agents, and approved drugs. BSAs work by inhibiting viral proteins or by targeting host cell factors and processes exploited by different viruses during infection. As of 2021, there are 150 known BSAs in varying stages of development, effective against 78 human viruses. BSAs are potential candidates for treatment of emerging and re-emerging viruses, such as ebola, marburg, and SARS-CoV-2. Many BSAs show antiviral activity against other viruses than originally investigated. Efforts in drug repurposing for SARS-CoV-2 is currently underway. A database of BSAs and viruses they inhibit could be found here.
References
- ↑ "NIH funds development of new broad-spectrum therapeutics". National Institutes of Health (NIH). 2015-09-18. Retrieved 2020-09-14.
- ↑ "Biodefense Strategic Plan | NIH: National Institute of Allergy and Infectious Diseases". www.niaid.nih.gov. Retrieved 2020-09-14.