In chemistry, a hydrate is a substance that contains water or its constituent elements. The chemical state of the water varies widely between different classes of hydrates, some of which were so labeled before their chemical structure was understood.

Magnesium sulfate or magnesium sulphate (in English-speaking countries other than the US) is a chemical compound, a salt with the formula MgSO4, consisting of magnesium cations Mg2+ (20.19% by mass) and sulfate anions SO2−4. It is a white crystalline solid, soluble in water but not in ethanol.

Chloral hydrate is a geminal diol with the formula C2H3Cl3O2. It is a colorless solid. It has limited use as a sedative and hypnotic pharmaceutical drug. It is also a useful laboratory chemical reagent and precursor. It is derived from chloral (trichloroacetaldehyde) by the addition of one equivalent of water.

Hydromorphone, also known as dihydromorphinone, and sold under the brand name Dilaudid among others, is a morphinan opioid used to treat moderate to severe pain. Typically, long-term use is only recommended for pain due to cancer. It may be used by mouth or by injection into a vein, muscle, or under the skin. Effects generally begin within half an hour and last for up to five hours.
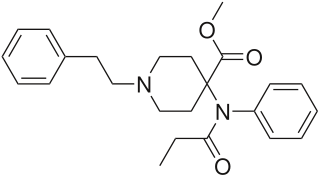
Carfentanil or carfentanyl, sold under the brand name Wildnil, is an extremely potent opioid analgesic which is used in veterinary medicine to anesthetize large animals such as elephants and rhinoceroses. It is typically administered in this context by tranquilizer dart. Carfentanil has also been used in humans for imaging of opioid receptors. It has additionally been used as a recreational drug, typically by injection, insufflation, or inhalation. Deaths have been reported in association with carfentanil.
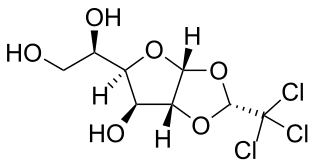
Chloralose is an avicide, and a rodenticide used to kill mice in temperatures below 15 °C. It is also widely used in neuroscience and veterinary medicine as an anesthetic and sedative. Either alone or in combination, such as with urethane, it is used for long-lasting, but light anesthesia.

Phenazone is an analgesic, antipyretic and anti-inflammatory drug. While it predates the term, it is often classified as a nonsteroidal anti-inflammatory drug (NSAID). Phenazone was one of the earliest synthetic medications — when it was patented in 1883, the only synthetic medical chemicals on the market were chloral hydrate, a sedative, trimethylamine, and iodol (tetraiodopyrrol), an early antiseptic. One of the earliest widely used analgesics and antipyretics, phenazone was gradually replaced in common use by other medications including phenacetin, aspirin, paracetamol and modern NSAIDs such as ibuprofen. However, it is still available in several countries either as an over-the-counter or prescribed drug.
Chloral, also known as trichloroacetaldehyde or trichloroethanal, is the organic compound with the formula Cl3CCHO. This aldehyde is a colourless liquid that is soluble in a wide range of solvents. It reacts with water to form chloral hydrate, a once widely used sedative and hypnotic substance.

Metamizole, or dipyrone, is a painkiller, spasm reliever, and fever reliever. It is most commonly given by mouth or by intravenous infusion. It belongs to the ampyrone sulfonate family of medicines and was patented in 1922. Metamizole is marketed under various trade names. It was first used medically in Germany under the brandname "Novalgin".

Tetracaine, also known as amethocaine, is an ester local anesthetic used to numb the eyes, nose, or throat. It may also be applied to the skin before starting an intravenous (injection) to decrease pain from the procedure. Typically it is applied as a liquid to the area. Onset of effects when used in the eyes is within 30 seconds and last for less than 15 minutes.
Melzer's reagent is a chemical reagent used by mycologists to assist with the identification of fungi, and by phytopathologists for fungi that are plant pathogens.

Ohmefentanyl is an extremely potent opioid analgesic drug which selectively binds to the µ-opioid receptor.

Petrichloral is a sedative and hypnotic chloral hydrate prodrug. It is a Schedule IV drug in the USA.

Nefopam, sold under the brand name Acupan among others, is a centrally acting, non-opioid painkilling medication, that is primarily used to treat moderate to severe pain.

Taltirelin is a thyrotropin-releasing hormone (TRH) analog, which mimics the physiological actions of TRH, but with a much longer half-life and duration of effects, and little development of tolerance following prolonged dosing. It has nootropic, neuroprotective and analgesic effects.
Archibald Magill Fauntleroy was a physician. He graduated in medicine at the University of Pennsylvania in 1856, and in 1857 entered the United States Army as assistant surgeon; but, upon the start of the Civil War, he and his brother, a lieutenant in the navy, resigned at the same time with their father, Thomas T. Fauntleroy. He became a surgeon in the Confederate army, and was president of the board for the admission of surgeons, and chief officer on the medical staff of Gen. Joseph E. Johnston, and served with him until the battle of Seven Pines. He was then ordered to build and organize the hospitals at Danville, Virginia, and afterward had charge of the military hospital at Staunton, Virginia, until the war ended. He remained and practised at Staunton after the war, and was for several years superintendent of the lunatic asylum at that place. His contributions to medical literature include papers on potassium bromide, chloral hydrate, the use of chloroform in obstetrical practice, and a “Report upon Advance in Therapeutics,” which was printed in the Transactions of the Virginia Medical Society.

Tolibut, also known as 3-(p-tolyl)-4-aminobutyric acid (or β-(4-methylphenyl)-GABA), is drug that was developed in Russia. It is an analogue of γ-aminobutyric acid (GABA) (that is, a GABA analogue) and is the 4-methyl analogue of phenibut, and is also an analogue of baclofen where the 4-chloro substitution has been replaced with a 4-methyl substitution. Tolibut has been described as possessing analgesic, tranquilizing, and neuroprotective properties. It is not fully clear as to whether the drug was ever approved or used medically in Russia, though it may have been.

2,2,2-Tribromoethanol, often called just tribromoethanol, is a chemical compound with formula Br3C−CH2OH. Its molecule can be described as that of ethanol, with the three hydrogen atoms in position 2 replaced by bromine. It is a white crystalline solid, soluble in water and other solvents, that absorbs strongly in the UV below 290 nm.

Chloral cyanohydrin is the cyanohydrin derivative of chloral (trichloroacetaldehyde). It was historically used as a source of hydrogen cyanide for medicinal purposes. Chloral cyanohydrin is toxic by inhalation.

Bromal (tribromoacetaldehyde) is a brominated aldehyde. It reacts with water to form bromal hydrate.


















