In chemistry, biochemistry, and pharmacology, a dissociation constant is a specific type of equilibrium constant that measures the propensity of a larger object to separate (dissociate) reversibly into smaller components, as when a complex falls apart into its component molecules, or when a salt splits up into its component ions. The dissociation constant is the inverse of the association constant. In the special case of salts, the dissociation constant can also be called an ionization constant.
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal–ligand bonding can range from covalent to ionic. Furthermore, the metal–ligand bond order can range from one to three. Ligands are viewed as Lewis bases, although rare cases are known to involve Lewis acidic "ligands".
Chelation is a type of bonding of ions and molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate ligand and a single central atom. These ligands are called chelants, chelators, chelating agents, or sequestering agents. They are usually organic compounds.
The pseudohalogens are polyatomic analogues of halogens, whose chemistry, resembling that of the true halogens, allows them to substitute for halogens in several classes of chemical compounds. Pseudohalogens occur in pseudohalogen molecules, inorganic molecules of the general forms Ps–Ps or Ps–X, such as cyanogen; pseudohalide anions, such as cyanide ion; inorganic acids, such as hydrogen cyanide; as ligands in coordination complexes, such as ferricyanide; and as functional groups in organic molecules, such as the nitrile group. Well-known pseudohalogen functional groups include cyanide, cyanate, thiocyanate, and azide.
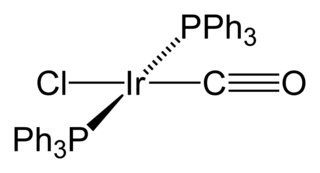
Vaska's complex is the trivial name for the chemical compound trans-carbonylchlorobis(triphenylphosphine)iridium(I), which has the formula IrCl(CO)[P(C6H5)3]2. This square planar diamagnetic organometallic complex consists of a central iridium atom bound to two mutually trans triphenylphosphine ligands, carbon monoxide, and a chloride ion. The complex was first reported by J. W. DiLuzio and Lauri Vaska in 1961. Vaska's complex can undergo oxidative addition and is notable for its ability to bind to O2 reversibly. It is a bright yellow crystalline solid.

Tetrafluoroborate is the anion BF−
4. This tetrahedral species is isoelectronic with tetrafluoroberyllate (BeF2−
4), tetrafluoromethane (CF4), and tetrafluoroammonium (NF+
4) and is valence isoelectronic with many stable and important species including the perchlorate anion, ClO−
4, which is used in similar ways in the laboratory. It arises by the reaction of fluoride salts with the Lewis acid BF3, treatment of tetrafluoroboric acid with base, or by treatment of boric acid with hydrofluoric acid.

Gold(III) bromide is a dark-red to black crystalline solid. It has the empirical formula AuBr3, but exists primarily as a dimer with the molecular formula Au2Br6 in which two gold atoms are bridged by two bromine atoms. It is commonly referred to as gold(III) bromide, gold tribromide, and rarely but traditionally auric bromide, and sometimes as digold hexabromide. As is similar with the other gold halides, this compound is unique for being a coordination complex of a group 11 transition metal that is stable in an oxidation state of three whereas copper or silver complexes persist in oxidation states of one or two.
The 18-electron rule is a rule used primarily for predicting and rationalizing formulas for stable metal complexes, especially organometallic compounds. The rule is based on the fact that the valence shells of transition metals consist of nine valence orbitals, which collectively can accommodate 18 electrons as either bonding or nonbonding electron pairs. This means that, the combination of these nine atomic orbitals with ligand orbitals creates nine molecular orbitals that are either metal-ligand bonding or non-bonding. When a metal complex has 18 valence electrons, it is said to have achieved the same electron configuration as the noble gas in the period. The rule and its exceptions are similar to the application of the octet rule to main group elements. The rule is not helpful for complexes of metals that are not transition metals, and interesting or useful transition metal complexes will violate the rule because of the consequences deviating from the rule bears on reactivity. The rule was first proposed by American chemist Irving Langmuir in 1921.
The retinoid X receptor (RXR) is a type of nuclear receptor that is activated by 9-cis retinoic acid, which is discussed controversially to be of endogenous relevance, and 9-cis-13,14-dihydroretinoic acid, which is likely to be the major endogenous mammalian RXR-selective agonist.

Tetraxenonogold(II), gold tetraxenide(II) or AuXe2+
4 is a cationic complex with a square planar configuration of atoms. It is found in the compound AuXe2+
4(Sb
2F−
11)
2, which exists in triclinic and tetragonal crystal modifications. The AuXe2+
4 ion is stabilised by interactions with the fluoride atoms of the counterion. The Au-Xe bond length is 274 pm = 2.74 angstroms.

Chloroauric acid is an inorganic compound with the chemical formula HAuCl
4. Both the trihydrate and tetrahydrate are known. It is an orange-yellow solid, a common precursor to other gold compounds and an intermediate in the purification of gold metal. Both the trihydrate and tetrahydrate are available commercially.
A migratory insertion is a type of reaction in organometallic chemistry wherein two ligands on a metal complex combine. It is a subset of reactions that very closely resembles the insertion reactions, and both are differentiated by the mechanism that leads to the resulting stereochemistry of the products. However, often the two are used interchangeably because the mechanism is sometimes unknown. Therefore, migratory insertion reactions or insertion reactions, for short, are defined not by the mechanism but by the overall regiochemistry wherein one chemical entity interposes itself into an existing bond of typically a second chemical entity e.g.:

Denticity refers to the number of donor groups in a single ligand that bind to a central atom in a coordination complex. In many cases, only one atom in the ligand binds to the metal, so the denticity equals one, and the ligand is said to be monodentate. Ligands with more than one bonded atom are called polydentate or multidentate. The word denticity is derived from dentis, the Latin word for tooth. The ligand is thought of as biting the metal at one or more linkage points. The denticity of a ligand is described with the Greek letter κ ('kappa'). For example, κ6-EDTA describes an EDTA ligand that coordinates through 6 non-contiguous atoms.
Organogold chemistry is the study of compounds containing gold–carbon bonds. They are studied in academic research, but have not received widespread use otherwise. The dominant oxidation states for organogold compounds are I with coordination number 2 and a linear molecular geometry and III with CN = 4 and a square planar molecular geometry. The first organogold compound discovered was gold(I) carbide Au2C2, which was first prepared in 1900.

Chloro(dimethyl sulfide)gold(I) is a coordination complex of gold. It is a white solid. This compound is a common entry point into gold chemistry.

Metal halides are compounds between metals and halogens. Some, such as sodium chloride are ionic, while others are covalently bonded. Covalently bonded metal halides may be discrete molecules, such as uranium hexafluoride, or they may form polymeric structures, such as palladium chloride.
In homogeneous catalysis, a C2-symmetric ligands usually describes bidentate ligands that are dissymmetric but not asymmetric by virtue of their C2-symmetry. Such ligands have proven valuable in catalysis. With C2 symmetry, C2-symmetric ligands limit the number of possible reaction pathways and thereby increase enantioselectivity, at least relative to asymmetrical analogues. Chiral ligands combine with metals to form chiral catalyst, which engages in a chemical reaction in which chirality is transfer to the reaction product. C2 symmetric ligands are a subset of chiral ligands.













