
Barium chloride is an inorganic compound with the formula BaCl2. It is one of the most common water-soluble salts of barium. Like most other water-soluble barium salts, it is a white powder, highly toxic, and imparts a yellow-green coloration to a flame. It is also hygroscopic, converting to the dihydrate BaCl2·2H2O, which are colourless crystals with a bitter salty taste. It has limited use in the laboratory and industry.
In chemistry, water(s) of crystallization or water(s) of hydration are water molecules that are present inside crystals. Water is often incorporated in the formation of crystals from aqueous solutions. In some contexts, water of crystallization is the total mass of water in a substance at a given temperature and is mostly present in a definite (stoichiometric) ratio. Classically, "water of crystallization" refers to water that is found in the crystalline framework of a metal complex or a salt, which is not directly bonded to the metal cation.

Sodium thiosulfate is an inorganic compound with the formula Na2S2O3·(H2O)(x) .Typically it is available as the white or colorless pentahydrate, It is a white solid that dissolves well in water. The compound is a reducing agent and a ligand, and these properties underpin its applications.

Manganese(II) chloride is the dichloride salt of manganese, MnCl2. This inorganic chemical exists in the anhydrous form, as well as the dihydrate (MnCl2·2H2O) and tetrahydrate (MnCl2·4H2O), with the tetrahydrate being the most common form. Like many Mn(II) species, these salts are pink, with the paleness of the color being characteristic of transition metal complexes with high spin d5 configurations.
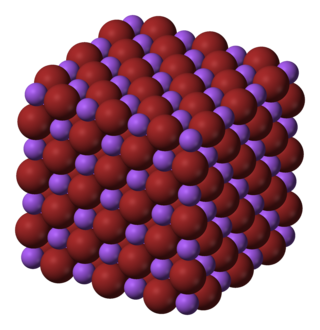
Sodium bromide is an inorganic compound with the formula NaBr. It is a high-melting white, crystalline solid that resembles sodium chloride. It is a widely used source of the bromide ion and has many applications.

Copper(II) chloride, also known as cupric chloride, is an inorganic compound with the chemical formula CuCl2. The monoclinic yellowish-brown anhydrous form slowly absorbs moisture to form the orthorhombic blue-green dihydrate CuCl2·2H2O, with two water molecules of hydration. It is industrially produced for use as a co-catalyst in the Wacker process.

Nickel(II) chloride (or just nickel chloride) is the chemical compound NiCl2. The anhydrous salt is yellow, but the more familiar hydrate NiCl2·6H2O is green. Nickel(II) chloride, in various forms, is the most important source of nickel for chemical synthesis. The nickel chlorides are deliquescent, absorbing moisture from the air to form a solution. Nickel salts have been shown to be carcinogenic to the lungs and nasal passages in cases of long-term inhalation exposure.
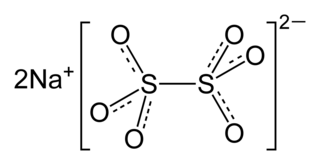
Sodium dithionate Na2S2O6 is an important compound for inorganic chemistry. It is also known under names disodium dithionate, sodium hyposulfate, and sodium metabisulfate. The sulfur can be considered to be in its +5 oxidation state.

Sodium molybdate, Na2MoO4, is useful as a source of molybdenum. This white, crystalline salt is often encountered as the dihydrate, Na2MoO4·2H2O.
Sodium orthovanadate is the inorganic compound with the chemical formula Na3V O4. It forms a dihydrate Na3VO4·2H2O. Sodium orthovanadate is a salt of the VO3−4 oxyanion. It is a colorless, water-soluble solid.

Vanadium(III) chloride describes the inorganic compound with the formula VCl3 and its hydrates. It forms a purple anhydrous form and a green hexahydrate [VCl2(H2O)4]Cl·2H2O. These hygroscopic salts are common precursors to other vanadium(III) complexes and is used as a mild reducing agent.

Monosodium phosphate (MSP), also known as monobasic sodium phosphate and sodium dihydrogen phosphate, is an inorganic compound with the chemical formula NaH2PO4. It is a sodium salt of phosphoric acid. It consists of sodium cations (Na+) and dihydrogen phosphate anions (H2PO−4). One of many sodium phosphates, it is a common industrial chemical. The salt exists in an anhydrous form, as well as monohydrate and dihydrate (NaH2PO4·H2O and NaH2PO4·2H2O respectively).

Chloroauric acid is an inorganic compound with the chemical formula H[AuCl4]. It forms hydrates H[AuCl4]·nH2O. Both the trihydrate and tetrahydrate are known. Both are orange-yellow solids consisting of the planar [AuCl4]− anion. Often chloroauric acid is handled as a solution, such as those obtained by dissolution of gold in aqua regia. These solutions can be converted to other gold complexes or reduced to metallic gold or gold nanoparticles.
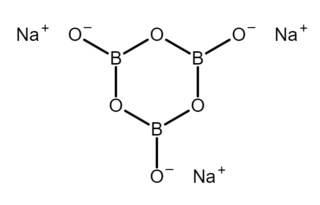
Sodium metaborate is a chemical compound of sodium, boron, and oxygen with formula NaBO2. However, the metaborate ion is trimeric in the anhydrous solid, therefore a more correct formula is Na3B3O6 or (Na+)3[B3O6]3−. The formula can be written also as Na2O·B2O3 to highlight the relation to the main oxides of sodium and boron. The name is also applied to several hydrates whose formulas can be written NaBO2·nH2O for various values of n.

Croconic acid is a chemical compound with formula C5H2O5 or (C=O)3(COH)2. It has a cyclopentene backbone with two hydroxyl groups adjacent to the double bond and three ketone groups on the remaining carbon atoms. It is sensitive to light, soluble in water and ethanol and forms yellow crystals that decompose at 212 °C.

Ammonium thiosulfate is an inorganic compound with the formula [NH4]2S2O3. It is white crystalline solid with ammonia odor, readily soluble in water, slightly soluble in acetone and insoluble in ethanol and diethyl ether.

Cerium nitrate refers to a family of nitrates of cerium in the +3 or +4 oxidation state. Often these compounds contain water, hydroxide, or hydronium ions in addition to cerium and nitrate. Double nitrates of cerium also exist.

The Nickel oxyacid salts are a class of chemical compounds of nickel with an oxyacid. The compounds include a number of minerals and industrially important nickel compounds.
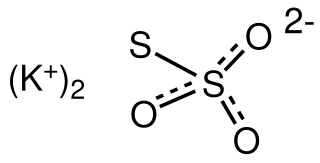
Potassium thiosulfate, commonly abbreviated KTS, is an inorganic compound with the formula K2S2O3. This salt can form multiple hydrates, such as the monohydrate, dihydrate, and the pentahydrate, all of which are white or colorless solids. It is used as a fertilizer.

A transition metal thiosulfate complex is a coordination complex containing one or more thiosulfate ligands. Thiosulfate occurs in nature and is used industrially, so its interactions with metal ions are of some practical interest. Three binding modes are common: monodentate (κ1-), O,S-bidentate (κ2-),and bridging (μ-). Typically, thiosulfate complexes are prepared from thiosulfate salts. In some cases, they arise by oxidation of polysulfido complexes, or by binding of sulfur trioxide to sulfido ligands.

![X-ray crystallographic structure of
Na3[Au(S2O3)2]*2H2O. Color code: red = O, orange = Au, yellow = S, violet = Na. Hydrogen atoms are omitted. Sodium-gold(I)-thiosulfate-dihydrate-unit-cell-3D-balls.png](http://upload.wikimedia.org/wikipedia/commons/thumb/9/9e/Sodium-gold%28I%29-thiosulfate-dihydrate-unit-cell-3D-balls.png/220px-Sodium-gold%28I%29-thiosulfate-dihydrate-unit-cell-3D-balls.png)


















