A bark beetle is the common name for the subfamily of beetles Scolytinae. Previously, this was considered a distinct family (Scolytidae), but is now understood to be a specialized clade of the "true weevil" family (Curculionidae). Although the term "bark beetle" refers to the fact that many species feed in the inner bark (phloem) layer of trees, the subfamily also has many species with other lifestyles, including some that bore into wood, feed in fruit and seeds, or tunnel into herbaceous plants. Well-known species are members of the type genus Scolytus, namely the European elm bark beetle S. multistriatus and the large elm bark beetle S. scolytus, which like the American elm bark beetle Hylurgopinus rufipes, transmit Dutch elm disease fungi (Ophiostoma). The mountain pine beetle Dendroctonus ponderosae, southern pine beetle Dendroctonus frontalis, and their near relatives are major pests of conifer forests in North America. A similarly aggressive species in Europe is the spruce ips Ips typographus. A tiny bark beetle, the coffee berry borer, Hypothenemus hampei is a major pest on coffee plantations around the world.

Oak wilt is a fungal disease caused by the organism Bretziella fagacearum that threatens Quercus spp. The disease is limited to the eastern half of the United States; first described in the 1940s in the Upper Mississippi River Valley. The pathogen penetrates xylem tissue, preventing water transport and causing disease symptoms. Symptoms generally consist of leaf discoloration, wilt, defoliation, and death. The disease is dispersed by insect vectors and to adjacent trees through underground root networks. However, human spread is the most consequential dispersal method. Moving firewood long distances can potentially transport diseases and invasive species.

Pinus thunbergii, the black pine, Japanese black pine, or Japanese pine, is a pine tree native to coastal areas of Japan and South Korea.

Stilbenoids are hydroxylated derivatives of stilbene. They have a C6–C2–C6 structure. In biochemical terms, they belong to the family of phenylpropanoids and share most of their biosynthesis pathway with chalcones. Most stilbenoids are produced by plants, and the only known exception is the antihelminthic and antimicrobial stilbenoid, 2-isopropyl-5-[(E)-2-phenylvinyl]benzene-1,3-diol, biosynthesized by the Gram-negative bacterium Photorhabdus luminescens.

Ophiostoma ulmi is a species of fungus in the family Ophiostomataceae. It is one of the causative agents of Dutch elm disease. It was first described under the name Graphium ulmi, and later transferred to the genus Ophiostoma.

Monochamus is a genus of longhorn beetles found throughout the world. They are commonly known as sawyer beetles or sawyers, as their larvae bore into dead or dying trees, especially conifers such as pines. They are the type genus of the Monochamini, a tribe in the huge long-horned beetle subfamily Lamiinae, but typically included in the Lamiini today.

Bursaphelenchus is a genus of nematodes (roundworms) in the order Aphelenchida. Most are obligate mycophages, but some feed on wood, with two species, the red ring nematode and the pine wood nematode, economically significant as pests of coconut palms and of pine trees, respectively. Given that Bursaphelenchus species are usually hard to distinguish from one another except by trained nematologists with access to microscopes or DNA sequence analysis, the entire genus is put under quarantine in some countries. Where this is not the case however, these nematodes are becoming established as model organisms for nematode developmental biology, ecology and genetics.
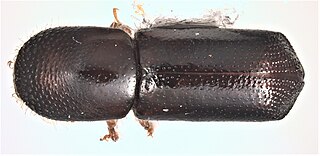
Xyleborus glabratus, the redbay ambrosia beetle, is a type of ambrosia beetle invasive in the United States. It has been documented as the primary vector of Raffaelea lauricola, the fungus that causes laurel wilt, a disease that can kill several North American tree species in the family Lauraceae, including redbay, sassafras, and avocado.
The red ring disease of coconuts and African oil palms is caused by the nematode Bursaphelenchus cocophilus. It is also identified in literature with an alternative scientific name Rhadinaphelenchus cocophilus. The common name, the red ring nematode, is derived from its distinguishing symptom.

A wilt disease is any number of diseases that affect the vascular system of plants. Attacks by fungi, bacteria, and nematodes can cause rapid killing of plants, large tree branches or even entire trees.
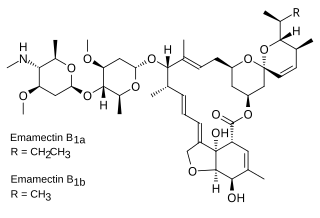
Emamectin is the 4″-deoxy-4″-methylamino derivative of abamectin, a 16-membered macrocyclic lactone produced by the fermentation of the soil actinomycete Streptomyces avermitilis. It is generally prepared as the salt with benzoic acid, emamectin benzoate, which is a white or faintly yellow powder. Emamectin is widely used in the US and Canada as an insecticide because of its chloride channel activation properties.
Forest pathology is the research of both biotic and abiotic maladies affecting the health of a forest ecosystem, primarily fungal pathogens and their insect vectors. It is a subfield of forestry and plant pathology.

Fusarium circinatum is a fungal plant pathogen that causes the serious disease pitch canker on pine trees and Douglas firs. The most common hosts of the pathogen include slash pine, loblolly pine, Monterey pine, Mexican weeping pine, and Douglas fir. Like other Fusarium species in the phylum Ascomycota, it is the asexual reproductive state of the fungus and has a teleomorph, Gibberella circinata.

Lira is a village (aldea) belonging to the O Condado shire, in Galicia, north west of Spain. Legally is one of the parishes (parroquia) under the government of the council of Salvaterra de Miño. It had 327 inhabitants in 2004 divided into 15 small villages (lugares). The patron saint of the parish is Saint Simon.

Monochamus scutellatus, commonly known as the white-spotted sawyer or spruce sawyer or spruce bug, is a common wood-boring beetle found throughout North America. It is a species native to North America.
Japanese oak wilt is a fungal disease caused by Raffaelea quercivora fungus affecting by oak trees. In 1998, Japanese plant pathologists group was isolation, inoculation and reisolation the dead tree. It is the first disease known that Raffaela fungus cause plant disease.

Monochamus subfasciatus is a species of beetle in the family Cerambycidae. It was described by Henry Walter Bates in 1873. It is recorded from Japan where it infests Japanese red pine and is a vector of the nematode Bursaphelenchus doui.

Monochamus alternatus, the Japanese pine sawyer, is a species of beetle in the family Cerambycidae. It was described by Frederick William Hope in 1842. It is known from Hong Kong, Vietnam, Laos, North Korea, South Korea, Japan, China, and Taiwan. It feeds on Pinus banksiana, Abies firma, Pinus armandii, Pinus massoniana, Pinus yunnanensis, and Pinus densiflora. It serves as a vector for the nematode Bursaphelenchus xylophilus.

Monochamus galloprovincialis, the pine sawyer beetle, also referred to as the black pine sawyer beetle, is a species of beetle in the family Cerambycidae. It was described by Olivier in 1795, originally under the genus Cerambyx. It has a wide distribution, occurring naturally throughout Europe and the Caucasus. It has also been introduced into the Canary Islands. It serves as a vector for the parasitic nematode species Bursaphelenchus xylophilus, and also acts as a host to the parasitoid wasp species Dolichomitus tuberculatus.

Monochamus sartor urussovii is a subspecies of beetle in the family Cerambycidae. It was described by Fischer von Waldheim in 1806. It has been recorded in Eastern and Northern Europe and Northern Asia. It is a vector of the nematode Bursaphelenchus mucronatus. Larvae burrow into the wood of various conifer species and can be a tree pest, as feeding damage reduces the value of the timber.