
Club drugs, also called rave drugs or party drugs, are a loosely defined category of recreational drugs which are associated with discothèques in the 1970s and nightclubs, dance clubs, electronic dance music (EDM) parties, and raves in the 1980s to today. Unlike many other categories, such as opiates and benzodiazepines, which are established according to pharmaceutical or chemical properties, club drugs are a "category of convenience", in which drugs are included due to the locations they are consumed and/or where the user goes while under the influence of the drugs. Club drugs are generally used by adolescents and young adults.

Benzylpiperazine (BZP) is a substance often used as a recreational drug and is known to have euphoriant and stimulant properties. Several studies conducted between 2000 and 2011 found that the effects of BZP are similar to amphetamine, although BZP's dosage is roughly 10 times higher by weight.
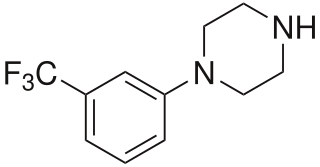
3-Trifluoromethylphenylpiperazine (TFMPP) is a recreational drug of the phenylpiperazine chemical class and is a substituted piperazine. Usually in combination with benzylpiperazine (BZP) and other analogues, it is sold as an alternative to the illicit drug MDMA ("Ecstasy").

Piperazine is an organic compound that consists of a six-membered ring containing two nitrogen atoms at opposite positions in the ring. Piperazine exists as small alkaline deliquescent crystals with a saline taste.
Sigma receptors (σ-receptors) are protein receptors that bind ligands such as 4-PPBP, SA 4503 (cutamesine), ditolylguanidine, dimethyltryptamine, and siramesine. There are two subtypes, sigma-1 receptors (σ1) and sigma-2 receptors (σ2), which are classified as sigma receptors for their pharmacological similarities, even though they are evolutionarily unrelated.

Vanoxerine is an investigational drug which is being evaluated for the treatment of heart arrhythmias and cocaine dependence. Vanoxerine is a piperazine derivative which has multiple pharmacological activities including acting as an dopamine reuptake inhibitor, serotonin transporter inhibitor, and as a blocker of the cardiac hERG repolarizing potassium channel (IKr).

para-Methoxyphenylpiperazine (pMeOPP), also known as 4-methoxyphenylpiperazine (4-MeOPP), is a substituted piperazine derivative with stimulant effects which has been sold as an ingredient in "Party pills", initially in New Zealand and subsequently in other countries around the world.

para-Fluorophenylpiperazine is a piperazine derivative with mildly psychedelic and euphoriant effects. It has been sold as an ingredient in legal recreational drugs known as "Party pills", initially in New Zealand and subsequently in other countries around the world.

Piberaline is a psychoactive drug and member of the piperazine chemical class which was developed in the 1980s. It has stimulant and antidepressant effects which are thought to be due largely to its active metabolite benzylpiperazine. It was studied to a limited extent in Hungary and Spain, but was not widely accepted and does not seem to be in current use, although a closely related drug befuraline with similar effects has been slightly more successful.

Methylbenzylpiperazine is a stimulant drug which is a derivative of benzylpiperazine. MBZP has been sold as an ingredient in legal recreational drugs known as "party pills", initially in New Zealand and subsequently in other countries around the world.
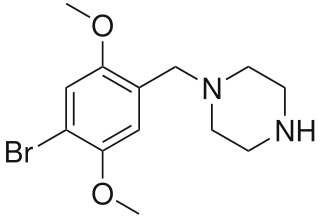
4-Bromo-2,5-dimethoxy-1-benzylpiperazine (2C-B-BZP) is a psychoactive drug and research chemical of the piperazine chemical class which has been sold as a "designer drug". It produces stimulant effects similar to those of benzylpiperazine (BZP).
Cocaine dependence is a neurological disorder that is characterized by withdrawal symptoms upon cessation from cocaine use. It also often coincides with cocaine addiction which is a biopsychosocial disorder characterized by persistent use of cocaine and/or crack despite substantial harm and adverse consequences. The Diagnostic and Statistical Manual of Mental Disorders, classifies problematic cocaine use as a stimulant use disorder. The International Classification of Diseases, includes "Cocaine dependence" as a classification (diagnosis) under "Disorders due to use of cocaine".
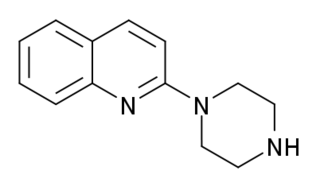
Quipazine, also known as 1-(2-quinolinyl)piperazine, is a serotonergic drug of the arylpiperazine family and an analogue of 1-(2-pyridinyl)piperazine which is used in scientific research. It was first described in the 1960s and was originally intended as an antidepressant but was never developed or marketed for medical use.

Clocinizine is a first-generation antihistamine of the diphenylmethylpiperazine class. It is marketed in Spain in combination with phenylpropanolamine under the brand name Senioral.

para-Chlorophenylpiperazine (pCPP) is a psychoactive drug of the phenylpiperazine class. It is relatively obscure, with limited human use, and produces slightly psychedelic effects. It has been encountered in illicit capsules as a recreational drug similarly to other piperazines like mCPP. Scientific research has demonstrated pCPP to have serotonergic effects, likely acting as a non-selective serotonin receptor agonist and/or releasing agent.

1-(2-Pyrimidinyl)piperazine (1-PP, 1-PmP) is a chemical compound and piperazine derivative. It is known to act as an antagonist of the α2-adrenergic receptor (Ki = 7.3–40 nM) and, to a much lesser extent, as a partial agonist of the 5-HT1A receptor (Ki = 414 nM; Emax = 54%). It has negligible affinity for the dopamine D2, D3, and D4 receptors (Ki > 10,000 nM) and does not appear to have significant affinity for the α1-adrenergic receptors. Its crystal structure has been determined.

Osemozotan (MKC-242) is a selective 5-HT1A receptor agonist with some functional selectivity, acting as a full agonist at presynaptic and a partial agonist at postsynaptic 5-HT1A receptors. 5-HT1A receptor stimulation influences the release of various neurotransmitters including serotonin, dopamine, norepinephrine, and acetylcholine. 5-HT1A receptors are inhibitory G protein-coupled receptor.

MT-45 (IC-6) is an opioid analgesic drug invented in the 1970s by Dainippon Pharmaceutical Co. It is chemically a 1-substituted-4-(1,2-diphenylethyl) piperazine derivative, which is structurally unrelated to most other opioid drugs. Racemic MT-45 has around 80% the potency of morphine, with almost all opioid activity residing in the (S) enantiomer. It has been used as a lead compound from which a large family of potent opioid drugs have been developed, including full agonists, partial agonists, and antagonists at the three main opioid receptor subtypes. Fluorinated derivatives of MT-45 such as 2F-MT-45 are significantly more potent as μ-opioid receptor agonists, and one of its main metabolites 1,2-diphenylethylpiperazine also blocks NMDA receptors.

1-(3-Chlorophenyl)-4-(2-phenylethyl)piperazine (3C-PEP) is a designer drug of the piperazine class of chemical substances. 3C-PEP is related to meta-cholorophenylpiperazine (mCPP) and phenethylamine that can be thought of as mCPP having a phenylethyl group attached to the nitrogen atom at its 4-position. It was first described in 1994 in a patent disclosing a series of piperazine compounds as sigma receptor ligands. Later, it was discovered to be a highly potent dopamine reuptake inhibitor.

ortho-Methylphenylpiperazine (also known as oMPP, oMePP, 1-(2-methylphenyl)piperazine, 2-MPP, and 2-MePP) is a psychoactive designer drug of the phenylpiperazine group. It acts as a serotonin–norepinephrine–dopamine releasing agent (SNDRA), with EC50 values for induction of monoamine release of 175 nM for serotonin, 39.1 nM for norepinephrine, and 296–542 nM for dopamine. As such, it has about 4.5-fold preference for induction of norepinephrine release over serotonin, and about 7.6- to 13.9-fold preference for induction of norepinephrine release over dopamine.


















