
Calcium is a chemical element with the symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to its heavier homologues strontium and barium. It is the fifth most abundant element in Earth's crust, and the third most abundant metal, after iron and aluminium. The most common calcium compound on Earth is calcium carbonate, found in limestone and the fossilised remnants of early sea life; gypsum, anhydrite, fluorite, and apatite are also sources of calcium. The name derives from Latin calx "lime", which was obtained from heating limestone.

The citric acid cycle (CAC)—also known as the Krebs cycle or the TCA cycle —is a series of chemical reactions to release stored energy through the oxidation of acetyl-CoA derived from carbohydrates, fats, and proteins. The Krebs cycle is used by organisms that respire to generate energy, either by anaerobic respiration or aerobic respiration. In addition, the cycle provides precursors of certain amino acids, as well as the reducing agent NADH, that are used in numerous other reactions. Its central importance to many biochemical pathways suggests that it was one of the earliest components of metabolism and may have originated abiogenically. Even though it is branded as a 'cycle', it is not necessary for metabolites to follow only one specific route; at least three alternative segments of the citric acid cycle have been recognized.

Calcium carbonate is a chemical compound with the formula CaCO3. It is a common substance found in rocks as the minerals calcite and aragonite and is the main component of eggshells, gastropod shells, shellfish skeletons and pearls. Things containing much calcium carbonate or resembling it are described as calcareous. Calcium carbonate is the active ingredient in agricultural lime and is created when calcium ions in hard water react with carbonate ions to create limescale. It has medical use as a calcium supplement or as an antacid, but excessive consumption can be hazardous and cause hypercalcemia and digestive issues.
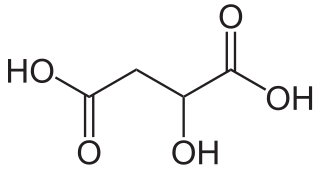
Malic acid is an organic compound with the molecular formula C4H6O5. It is a dicarboxylic acid that is made by all living organisms, contributes to the sour taste of fruits, and is used as a food additive. Malic acid has two stereoisomeric forms, though only the L-isomer exists naturally. The salts and esters of malic acid are known as malates. The malate anion is an intermediate in the citric acid cycle.

Oxaloacetic acid (also known as oxalacetic acid or OAA) is a crystalline organic compound with the chemical formula HO2CC(O)CH2CO2H. Oxaloacetic acid, in the form of its conjugate base oxaloacetate, is a metabolic intermediate in many processes that occur in animals. It takes part in gluconeogenesis, the urea cycle, the glyoxylate cycle, amino acid synthesis, fatty acid synthesis and the citric acid cycle.

Malate dehydrogenase (EC 1.1.1.37) (MDH) is an enzyme that reversibly catalyzes the oxidation of malate to oxaloacetate using the reduction of NAD+ to NADH. This reaction is part of many metabolic pathways, including the citric acid cycle. Other malate dehydrogenases, which have other EC numbers and catalyze other reactions oxidizing malate, have qualified names like malate dehydrogenase (NADP+).

Fumarase is an enzyme that catalyzes the reversible hydration/dehydration of fumarate to malate. Fumarase comes in two forms: mitochondrial and cytosolic. The mitochondrial isoenzyme is involved in the Krebs cycle and the cytosolic isoenzyme is involved in the metabolism of amino acids and fumarate. Subcellular localization is established by the presence of a signal sequence on the amino terminus in the mitochondrial form, while subcellular localization in the cytosolic form is established by the absence of the signal sequence found in the mitochondrial variety.
Calcium citrate malate is a water-soluble calcium supplement. It is the calcium salt of citric acid and malic acid with variable composition.

Guard cells are specialized plant cells in the epidermis of leaves, stems and other organs that are used to control gas exchange. They are produced in pairs with a gap between them that forms a stomatal pore. The stomatal pores are largest when water is freely available and the guard cells turgid, and closed when water availability is critically low and the guard cells become flaccid. Photosynthesis depends on the diffusion of carbon dioxide (CO2) from the air through the stomata into the mesophyll tissues. Oxygen (O2), produced as a byproduct of photosynthesis, exits the plant via the stomata. When the stomata are open, water is lost by evaporation and must be replaced via the transpiration stream, with water taken up by the roots. Plants must balance the amount of CO2 absorbed from the air with the water loss through the stomatal pores, and this is achieved by both active and passive control of guard cell turgor pressure and stomatal pore size.
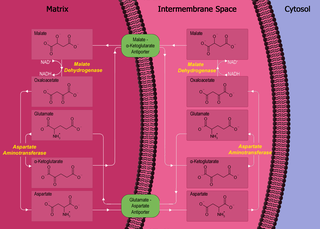
The malate-aspartate shuttle is a biochemical system for translocating electrons produced during glycolysis across the semipermeable inner membrane of the mitochondrion for oxidative phosphorylation in eukaryotes. These electrons enter the electron transport chain of the mitochondria via reduction equivalents to generate ATP. The shuttle system is required because the mitochondrial inner membrane is impermeable to NADH, the primary reducing equivalent of the electron transport chain. To circumvent this, malate carries the reducing equivalents across the membrane.
In enzymology, a D-malate dehydrogenase (decarboxylating) (EC 1.1.1.83) is an enzyme that catalyzes the chemical reaction
Malate dehydrogenase (decarboxylating) (EC 1.1.1.39) or NAD-malic enzyme (NAD-ME) is an enzyme that catalyzes the chemical reaction

In enzymology, a malate dehydrogenase (NADP+) (EC 1.1.1.82) is an enzyme that catalyzes the chemical reaction
In enzymology, a malate dehydrogenase (quinone), formerly malate dehydrogenase (acceptor), is an enzyme that catalyzes the chemical reaction

In enzymology, a malate synthase (EC 2.3.3.9) is an enzyme that catalyzes the chemical reaction

Calcium-binding mitochondrial carrier protein Aralar1 is a protein that in humans is encoded by the SLC25A12 gene. Aralar is an integral membrane protein located in the inner mitochondrial membrane. Its primary function as an antiporter is the transport of cytoplasmic glutamate with mitochondrial aspartate across the inner mitochondrial membrane, dependent on the binding of one calcium ion. Mutations in this gene cause early infantile epileptic encephalopathy 39 (EIEE39), symptomized by global hypomyelination of the central nervous system, refractory seizures, and neurodevelopmental impairment. This gene has connections to autism.

Cabozantinib, sold under the brand names Cometriq and Cabometyx among others, is a medication used to treat medullary thyroid cancer, renal cell carcinoma, and hepatocellular carcinoma. It is a small molecule inhibitor of the tyrosine kinases c-Met and VEGFR2, and also inhibits AXL and RET. It was discovered and developed by Exelixis Inc.

Remedios Circle, also known as the Plaza de la Virgen de los Remedios, Remedios Rotonda, and Rotary Circle, is a traffic circle in Malate, Manila in the Philippines, serving as the intersection between Remedios Street, Jorge Bocobo Street and Adriatico Street. The circle and a traversing street are both named after Nuestra Señora de los Remedios, the patroness of the nearby Malate Church, and is one of two major open spaces in Malate, the other being Plaza Rajah Sulayman.
Penicillium viticola is a species of fungus in the genus Penicillium which was isolated from grapes in Yamanashi Prefecture in Japan. Penicillium viticola produces calcium malate















