
Berkelium is a transuranic radioactive chemical element with the symbol Bk and atomic number 97. It is a member of the actinide and transuranium element series. It is named after the city of Berkeley, California, the location of the Lawrence Berkeley National Laboratory where it was discovered in December 1949. Berkelium was the fifth transuranium element discovered after neptunium, plutonium, curium and americium.

Curium is a transuranic, radioactive chemical element with the symbol Cm and atomic number 96. This actinide element was named after eminent scientists Marie and Pierre Curie, both known for their research on radioactivity. Curium was first intentionally made by the team of Glenn T. Seaborg, Ralph A. James, and Albert Ghiorso in 1944, using the cyclotron at Berkeley. They bombarded the newly discovered element plutonium with alpha particles. This was then sent to the Metallurgical Laboratory at University of Chicago where a tiny sample of curium was eventually separated and identified. The discovery was kept secret until after the end of World War II. The news was released to the public in November 1947. Most curium is produced by bombarding uranium or plutonium with neutrons in nuclear reactors – one tonne of spent nuclear fuel contains ~20 grams of curium.

Californium is a radioactive chemical element with the symbol Cf and atomic number 98. The element was first synthesized in 1950 at Lawrence Berkeley National Laboratory, by bombarding curium with alpha particles. It is an actinide element, the sixth transuranium element to be synthesized, and has the second-highest atomic mass of all elements that have been produced in amounts large enough to see with the naked eye. The element was named after the university and the U.S. state of California.

Einsteinium is a synthetic element with the symbol Es and atomic number 99. Einsteinium is a member of the actinide series and it is the seventh transuranium element. It was named in honor of Albert Einstein.

Few compounds of californium have been made and studied. The only californium ion that is stable in aqueous solutions is the californium(III) cation. The other two oxidation states are IV (strong oxidizing agents) and II (strong reducing agents). The element forms a water-soluble chloride, nitrate, perchlorate, and sulfate and is precipitated as a fluoride, oxalate or hydroxide. If problems of availability of the element could be overcome, then CfBr2 and CfI2 would likely be stable.

Berkelium forms a number of chemical compounds, where it normally exists in an oxidation state of +3 or +4, and behaves similarly to its lanthanide analogue, terbium. Like all actinides, berkelium easily dissolves in various aqueous inorganic acids, liberating gaseous hydrogen and converting into the trivalent oxidation state. This trivalent state is the most stable, especially in aqueous solutions, but tetravalent berkelium compounds are also known. The existence of divalent berkelium salts is uncertain and has only been reported in mixed lanthanum chloride-strontium chloride melts. Aqueous solutions of Bk3+ ions are green in most acids. The color of the Bk4+ ions is yellow in hydrochloric acid and orange-yellow in sulfuric acid. Berkelium does not react rapidly with oxygen at room temperature, possibly due to the formation of a protective oxide surface layer; however, it reacts with molten metals, hydrogen, halogens, chalcogens and pnictogens to form various binary compounds. Berkelium can also form several organometallic compounds.

Californium oxychloride is a radioactive salt first discovered in measurable quantities in 1960. It is composed of a single californium cation and oxychloride consisting of one chloride and one oxide anion. It was the first californium compound ever isolated.
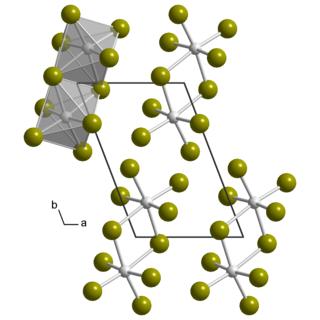
Uranium pentabromide is an inorganic chemical compound with the formula UBr5.

Curium(III) fluoride or curium trifluoride is the chemical compound composed of curium and fluorine with the formula CmF3. It is a white, nearly insoluble salt that has the same crystal structure as LaF3. It precipitates as a hydrate when fluoride ions are added to a weakly acidic Cm(III) solution; alternatively it can be synthesized by reacting hydrofluoric acid with Cm(OH)3. The anhydrous form is then obtained by desiccation or by treatment with hydrogen fluoride gas.
Oxybismuthides or bismuthide oxides are chemical compounds formally containing the group BiO, with one bismuth and one oxygen atom. The bismuth and oxygen are not bound together as in bismuthates, instead they make a separate presence bound to the cations (metals), and could be considered as a mixed bismuthide-oxide compound. So a compound with OmBin requires cations to balance a negative charge of 2m+3n. The cations will have charges of +2 or +3. The trications are often rare earth elements or actinides. They are in the category of oxypnictide compounds.
Neptunium (IV) oxalate is an inorganic compound, a salt of neptunium and oxalic acid with the chemical formula Np(C2O4)2. The compound is slightly soluble in water, forms crystalline hydrates—green crystals.
Curium(III) nitrate is an inorganic compound, a salt of curium and nitric acid with the chemical formula Cm(NO3)3.
Americium hexafluoride is an inorganic chemical compound of americium metal and fluorine with the chemical formula AmF
6. It is still a hypothetical compound. Synthesis by fluorination of americium tetrafluoride was unsuccessfully attempted in 1990. A thermochromatographic identification in 1986 remains inconclusive. Calculations suggest that it may be distorted from octahedral symmetry.
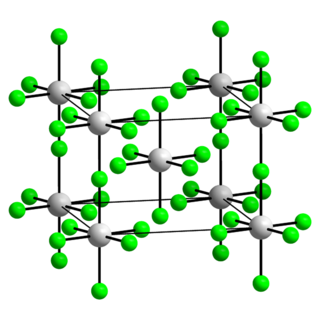
Plutonium pentafluoride is a binary inorganic compound of plutonium and fluorine with the chemical formula PuF5.
Californium(III) oxide is a binary inorganic compound of californium and oxygen with the formula Cf
2O
3. It is one of the first obtained solid compounds of californium, synthesized in 1958.

Berkelium(III) oxide is a binary inorganic compound of berkelium and oxygen with the chemical formula Bk
2O
3.

Berkelium bromide is a bromide of berkelium, with the chemical formula BkBr3.
Einsteinium fluoride is a binary inorganic chemical compound of einsteinium and fluorine with the chemical formula EsF3.
Einsteinium oxychloride is an inorganic chemical compound of einsteinium, oxygen, and chlorine with the chemical formula EsClO.
Californium(III) oxyiodide is a inorganic compound of californium, iodine, and oxygen with the formula CfOI.











