
An evaporite is a water-soluble sedimentary mineral deposit that results from concentration and crystallization by evaporation from an aqueous solution. There are two types of evaporite deposits: marine, which can also be described as ocean deposits, and non-marine, which are found in standing bodies of water such as lakes. Evaporites are considered sedimentary rocks and are formed by chemical sediments.

Magnesite is a mineral with the chemical formula MgCO
3. Iron, manganese, cobalt, and nickel may occur as admixtures, but only in small amounts.

The mineral marcasite, sometimes called "white iron pyrite", is iron sulfide (FeS2) with orthorhombic crystal structure. It is physically and crystallographically distinct from pyrite, which is iron sulfide with cubic crystal structure. Both structures contain the disulfide S22− ion, having a short bonding distance between the sulfur atoms. The structures differ in how these di-anions are arranged around the Fe2+ cations. Marcasite is lighter and more brittle than pyrite. Specimens of marcasite often crumble and break up due to the unstable crystal structure.

The sulfur cycle is a biogeochemical cycle in which the sulfur moves between rocks, waterways and living systems. It is important in geology as it affects many minerals and in life because sulfur is an essential element (CHNOPS), being a constituent of many proteins and cofactors, and sulfur compounds can be used as oxidants or reductants in microbial respiration. The global sulfur cycle involves the transformations of sulfur species through different oxidation states, which play an important role in both geological and biological processes. Steps of the sulfur cycle are:
Sulfur (16S) has 23 known isotopes with mass numbers ranging from 27 to 49, four of which are stable: 32S (95.02%), 33S (0.75%), 34S (4.21%), and 36S (0.02%). The preponderance of sulfur-32 is explained by its production from carbon-12 plus successive fusion capture of five helium-4 nuclei, in the so-called alpha process of exploding type II supernovas.
A paleothermometer is a methodology that provides an estimate of the ambient temperature at the time of formation of a natural material. Most paleothermometers are based on empirically-calibrated proxy relationships, such as the tree ring or TEX86 methods. Isotope methods, such as the δ18O method or the clumped-isotope method, are able to provide, at least in theory, direct measurements of temperature.

Oxygen isotope ratio cycles are cyclical variations in the ratio of the abundance of oxygen with an atomic mass of 18 to the abundance of oxygen with an atomic mass of 16 present in some substances, such as polar ice or calcite in ocean core samples, measured with the isotope fractionation. The ratio is linked to ancient ocean temperature which in turn reflects ancient climate. Cycles in the ratio mirror climate changes in the geological history of Earth.
γ-Carotene (gamma-carotene) is a carotenoid, and is a biosynthetic intermediate for cyclized carotenoid synthesis in plants. It is formed from cyclization of lycopene by lycopene cyclase epsilon. Along with several other carotenoids, γ-carotene is a vitamer of vitamin A in herbivores and omnivores. Carotenoids with a cyclized, beta-ionone ring can be converted to vitamin A, also known as retinol, by the enzyme beta-carotene 15,15'-dioxygenase; however, the bioconversion of γ-carotene to retinol has not been well-characterized. γ-Carotene has tentatively been identified as a biomarker for green and purple sulfur bacteria in a sample from the 1.640 ± 0.003-Gyr-old Barney Creek Formation in Northern Australia which comprises marine sediments. Tentative discovery of γ-carotene in marine sediments implies a past euxinic environment, where water columns were anoxic and sulfidic. This is significant for reconstructing past oceanic conditions, but so far γ-carotene has only been potentially identified in the one measured sample.

Mackinawite is an iron nickel sulfide mineral with the chemical formula (Fe,Ni)
1+xS. The mineral crystallizes in the tetragonal crystal system and has been described as a distorted, close packed, cubic array of S atoms with some of the gaps filled with Fe. Mackinawite occurs as opaque bronze to grey-white tabular crystals and anhedral masses. It has a Mohs hardness of 2.5 and a specific gravity of 4.17. It was first described in 1962 for an occurrence in the Mackinaw mine, Snohomish County, Washington for which it was named.
Professor Henry "Harry" Elderfield, was Professor of Ocean Chemistry and Palaeochemistry at the Godwin Laboratory in the Department of Earth Sciences at the University of Cambridge. He made his name in ocean chemistry and palaeochemistry, using trace metals and isotopes in biogenic carbonate as palaeochemical tracers, and studying the chemistry of modern and ancient oceans - especially those of the glacial epoch and the Cenozoic.
Clumped isotopes are heavy isotopes that are bonded to other heavy isotopes. The relative abundance of clumped isotopes (and multiply-substituted isotopologues) in molecules such as methane, nitrous oxide, and carbonate is an area of active investigation. The carbonate clumped-isotope thermometer, or "13C–18O order/disorder carbonate thermometer", is a new approach for paleoclimate reconstruction, based on the temperature dependence of the clumping of 13C and 18O into bonds within the carbonate mineral lattice. This approach has the advantage that the 18O ratio in water is not necessary (different from the δ18O approach), but for precise paleotemperature estimation, it also needs very large and uncontaminated samples, long analytical runs, and extensive replication. Commonly used sample sources for paleoclimatological work include corals, otoliths, gastropods, tufa, bivalves, and foraminifera. Results are usually expressed as Δ47 (said as "cap 47"), which is the deviation of the ratio of isotopologues of CO2 with a molecular weight of 47 to those with a weight of 44 from the ratio expected if they were randomly distributed.
The δ34S value is a standardized method for reporting measurements of the ratio of two stable isotopes of sulfur, 34S:32S, in a sample against the equivalent ratio in a known reference standard. Presently, the most commonly used standard is Vienna-Canyon Diablo Troilite (VCDT). Results are reported as variations from the standard ratio in parts per thousand, per mil or per mille, using the ‰ symbol. Heavy and light sulfur isotopes fractionate at different rates and the resulting δ34S values, recorded in marine sulfate or sedimentary sulfides, have been studied and interpreted as records of the changing sulfur cycle throughout the earth's history.
Isotopic reference materials are compounds with well-defined isotopic compositions and are the ultimate sources of accuracy in mass spectrometric measurements of isotope ratios. Isotopic references are used because mass spectrometers are highly fractionating. As a result, the isotopic ratio that the instrument measures can be very different from that in the sample's measurement. Moreover, the degree of instrument fractionation changes during measurement, often on a timescale shorter than the measurement's duration, and can depend on the characteristics of the sample itself. By measuring a material of known isotopic composition, fractionation within the mass spectrometer can be removed during post-measurement data processing. Without isotope references, measurements by mass spectrometry would be much less accurate and could not be used in comparisons across different analytical facilities. Due to their critical role in measuring isotope ratios, and in part, due to historical legacy, isotopic reference materials define the scales on which isotope ratios are reported in the peer-reviewed scientific literature.

Marine biogeochemical cycles are biogeochemical cycles that occur within marine environments, that is, in the saltwater of seas or oceans or the brackish water of coastal estuaries. These biogeochemical cycles are the pathways chemical substances and elements move through within the marine environment. In addition, substances and elements can be imported into or exported from the marine environment. These imports and exports can occur as exchanges with the atmosphere above, the ocean floor below, or as runoff from the land.
Euxinia or euxinic conditions occur when water is both anoxic and sulfidic. This means that there is no oxygen (O2) and a raised level of free hydrogen sulfide (H2S). Euxinic bodies of water are frequently strongly stratified, have an oxic, highly productive, thin surface layer, and have anoxic, sulfidic bottom water. The word euxinia is derived from the Greek name for the Black Sea (Εὔξεινος Πόντος (Euxeinos Pontos)) which translates to "hospitable sea". Euxinic deep water is a key component of the Canfield ocean, a model of oceans during the Proterozoic period (known as the Boring Billion) proposed by Donald Canfield, an American geologist, in 1998. There is still debate within the scientific community on both the duration and frequency of euxinic conditions in the ancient oceans. Euxinia is relatively rare in modern bodies of water, but does still happen in places like the Black Sea and certain fjords.
Shuhei Ono is a professor of earth, atmospheric, and planetary sciences at the Massachusetts Institute of Technology. In his research, he measures isotopes of sulfur and other elements to investigate water-rock-microbe interactions, seafloor hydrothermal systems, the deep biosphere, and global sulfur cycles.

Microbial oxidation of sulfur is the oxidation of sulfur by microorganisms to build their structural components. The oxidation of inorganic compounds is the strategy primarily used by chemolithotrophic microorganisms to obtain energy to survive, grow and reproduce. Some inorganic forms of reduced sulfur, mainly sulfide (H2S/HS−) and elemental sulfur (S0), can be oxidized by chemolithotrophic sulfur-oxidizing prokaryotes, usually coupled to the reduction of oxygen (O2) or nitrate (NO3−). Anaerobic sulfur oxidizers include photolithoautotrophs that obtain their energy from sunlight, hydrogen from sulfide, and carbon from carbon dioxide (CO2).
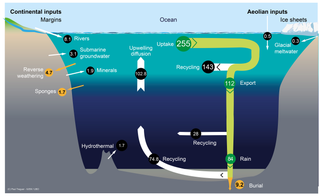
The silica cycle is the biogeochemical cycle in which biogenic silica is transported between the Earth's systems. Silicon is considered a bioessential element and is one of the most abundant elements on Earth. The silica cycle has significant overlap with the carbon cycle and plays an important role in the sequestration of carbon through continental weathering, biogenic export and burial as oozes on geologic timescales.
Sulfur isotope biogeochemistry is the study of the distribution of sulfur isotopes in biological and geological materials. In addition to its common isotope, 32S, sulfur has three rare stable isotopes: 34S, 36S, and 33S. The distribution of these isotopes in the environment is controlled by many biochemical and physical processes, including biological metabolisms, mineral formation processes, and atmospheric chemistry. Measuring the abundance of sulfur stable isotopes in natural materials, like bacterial cultures, minerals, or seawater, can reveal information about these processes both in the modern environment and over Earth history.
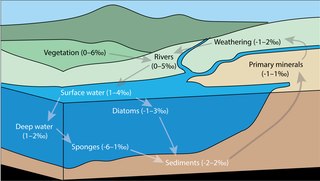
Silicon isotope biogeochemistry is the study of environmental processes using the relative abundance of Si isotopes. As the relative abundance of Si stable isotopes varies among different natural materials, the differences in abundance can be used to trace the source of Si, and to study biological, geological, and chemical processes. The study of stable isotope biogeochemistry of Si aims to quantify the different Si fluxes in the global biogeochemical silicon cycle, to understand the role of biogenic silica within the global Si cycle, and to investigate the applications and limitations of the sedimentary Si record as an environmental and palaeoceanographic proxy.











