Carbonic anhydrase 4 is an enzyme that in humans is encoded by the CA4 gene. [5] [6]
Carbonic anhydrase 4 is an enzyme that in humans is encoded by the CA4 gene. [5] [6]
Carbonic anhydrases (CAs) are a large family of zinc metalloenzymes that catalyze the reversible hydration of carbon dioxide. They participate in a variety of biological processes, including respiration, calcification, acid-base balance, bone resorption, and the formation of aqueous humor, cerebrospinal fluid, saliva, and gastric acid. They show extensive diversity in tissue distribution and in their subcellular localization. CA IV is a glycosylphosphatidyl-inositol-anchored membrane isozyme expressed on the luminal surfaces of pulmonary (and certain other) capillaries and of proximal renal tubules. Its exact function is not known, however, it may have a role in inherited renal abnormalities of bicarbonate transport. [6]
CA IV has been identified in pulmonary epithelium of many mammalian species and may be uniquely adaptive for gas exchange necessary for the high metabolic requirements of mammals. A majority of the CO2 produced by metabolism is transported as bicarbonate (HCO−
3). At the tissue capillary, CO2 diffuses from tissue to plasma. Other forms of carbonic anhydrase enzyme are not present in the plasma, restricting the equilibrium reaction of CO2+H2O = H
2CO
3 = H+ HCO−
3. CO2 in the plasma diffuses into the Red Blood Cell. CA is present within the Red Blood Cell, facilitating the conversion of CO2 to HCO−
3. HCO−
3 so produced is transferred by the HCO−
3/Cl- "shuttle" from the interior of the Red Blood Cell to the plasma. HCO−
3 does not diffuse across cell membranes and, in the absence of CA, stays as HCO−
3 and concentrates in plasma. Up to 80% of metabolically produced CO2 is transported in plasma in the form of HCO−
3. Blood moves from the tissue capillary to the pulmonary capillary where CO2 is exchanged at the lung. In the pulmonary capillary, bicarbonate can not simply diffuse either into the Red Blood Cell or the alveoli. It is traditionally thought that HCO−
3 is returned to the interior of the Red Blood Cell by a reversal of the HCO−
3/Cl- shuttle, where, in the presence of CA, it is returned to a CO2 form to diffuse from the interior of the Red Blood Cell, to the plasma and then into the alveoli. Membrane bound CA (CA IV) on the luminal side of the pulmonary membrane would have direct contact with plasma HCO−
3 and would enzymatically convert HCO−
3 to CO2 in the area immediately proximal to the exchange membrane, greatly increasing the concentration gradient for exchange. In this way, plasma HCO−
3 can be converted to CO2 within the plasma compartment and exchanged with the alveoli without the requirement of returning the HCO−
3 to the interior of the Red Blood Cell.

Red blood cells (RBCs), also referred to as red cells, red blood corpuscles (in humans or other animals not having nucleus in red blood cells), haematids, erythroid cells or erythrocytes (from Greek erythros 'red' and kytos 'hollow vessel', with -cyte translated as 'cell' in modern usage), are the most common type of blood cell and the vertebrate's principal means of delivering oxygen (O2) to the body tissues—via blood flow through the circulatory system. RBCs take up oxygen in the lungs, or in fish the gills, and release it into tissues while squeezing through the body's capillaries.

Acetazolamide, sold under the trade name Diamox among others, is a medication used to treat glaucoma, epilepsy, altitude sickness, periodic paralysis, idiopathic intracranial hypertension, heart failure and to alkalinize urine. It may be used long term for the treatment of open angle glaucoma and short term for acute angle closure glaucoma until surgery can be carried out. It is taken by mouth or injection into a vein. Acetazolamide is a first generation carbonic anhydrase inhibitor and it decreases the ocular fluid and osmolality in the eye to decrease intraocular pressure.
Gas exchange is the physical process by which gases move passively by diffusion across a surface. For example, this surface might be the air/water interface of a water body, the surface of a gas bubble in a liquid, a gas-permeable membrane, or a biological membrane that forms the boundary between an organism and its extracellular environment.
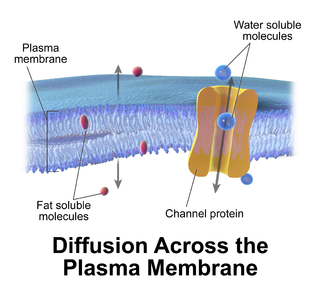
Passive transport is a type of membrane transport that does not require energy to move substances across cell membranes. Instead of using cellular energy, like active transport, passive transport relies on the second law of thermodynamics to drive the movement of substances across cell membranes. Fundamentally, substances follow Fick's first law, and move from an area of high concentration to one of low concentration because this movement increases the entropy of the overall system. The rate of passive transport depends on the permeability of the cell membrane, which, in turn, depends on the organization and characteristics of the membrane lipids and proteins. The four main kinds of passive transport are simple diffusion, facilitated diffusion, filtration, and/or osmosis.
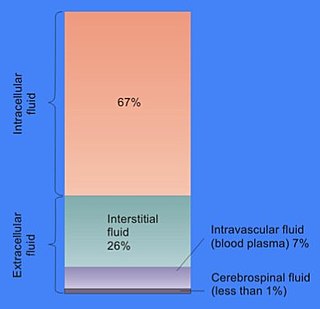
In cell biology, extracellular fluid (ECF) denotes all body fluid outside the cells of any multicellular organism. Total body water in healthy adults is about 60% of total body weight; women and the obese typically have a lower percentage than lean men. Extracellular fluid makes up about one-third of body fluid, the remaining two-thirds is intracellular fluid within cells. The main component of the extracellular fluid is the interstitial fluid that surrounds cells.

Renal physiology is the study of the physiology of the kidney. This encompasses all functions of the kidney, including maintenance of acid-base balance; regulation of fluid balance; regulation of sodium, potassium, and other electrolytes; clearance of toxins; absorption of glucose, amino acids, and other small molecules; regulation of blood pressure; production of various hormones, such as erythropoietin; and activation of vitamin D.
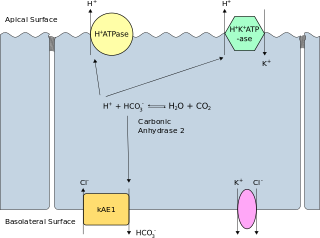
Band 3 anion transport protein, also known as anion exchanger 1 (AE1) or band 3 or solute carrier family 4 member 1 (SLC4A1), is a protein that is encoded by the SLC4A1 gene in humans.

Chloride shift (also known as the Hamburger phenomenon or lineas phenomenon, named after Hartog Jakob Hamburger) is a process which occurs in a cardiovascular system and refers to the exchange of bicarbonate (HCO3−) and chloride (Cl−) across the membrane of red blood cells (RBCs).
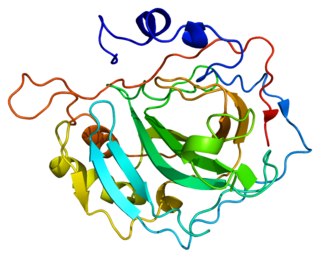
Carbonic anhydrase II, is one of sixteen forms of human α carbonic anhydrases. Carbonic anhydrase catalyzes reversible hydration of carbon dioxide. Defects in this enzyme are associated with osteopetrosis and renal tubular acidosis. Renal carbonic anhydrase allows the reabsorption of bicarbonate ions in the proximal tubule. Loss of carbonic anhydrase activity in bones impairs the ability of osteoclasts to promote bone resorption, leading to osteopetrosis.

Carbonic anhydrase 3 is an enzyme that in humans is encoded by the CA3 gene.
Carbonic anhydrase IX is an enzyme that in humans is encoded by the CA9 gene. It is one of the 14 carbonic anhydrase isoforms found in humans and is a transmembrane dimeric metalloenzyme with an extracellular active site that facilitates acid secretion in the gastrointestinal tract. CA IX is overexpressed in many types of cancer including clear cell renal cell carcinoma (RCC) as well as carcinomas of the cervix, breast and lung where it promotes tumor growth by enhancing tumor acidosis.

Electrogenic sodium bicarbonate cotransporter 1, sodium bicarbonate cotransporter is a membrane transport protein that in humans is encoded by the SLC4A4 gene.

Carbonic anhydrase 1 is an enzyme that in humans is encoded by the CA1 gene.

Carbonic anhydrase 12 is an enzyme that in humans is encoded by the CA12 gene.

Carbonic anhydrase 6 is an enzyme that in humans is encoded by the CA6 gene. It is also called 'gustin' because of its presence in saliva, and lower-than-normal levels of salivary zinc in individuals with hypogeusia.

Anion exchange protein 3 is a membrane transport protein that in humans is encoded by the SLC4A3 gene. AE3 is functionally similar to the Band 3 Cl−/HCO3− exchange protein but it is expressed primarily in brain neurons and in the heart. Like AE2 its activity is sensitive to pH. AE3 mutations have been linked to seizures.
Carbonic anhydrase 14 is an enzyme that in humans is encoded by the CA14 gene.

The carbonic anhydrases form a family of enzymes that catalyze the interconversion between carbon dioxide and water and the dissociated ions of carbonic acid. The active site of most carbonic anhydrases contains a zinc ion. They are therefore classified as metalloenzymes. The enzyme maintains acid-base balance and helps transport carbon dioxide.
The anion exchanger family is a member of the large APC superfamily of secondary carriers. Members of the AE family are generally responsible for the transport of anions across cellular barriers, although their functions may vary. All of them exchange bicarbonate. Characterized protein members of the AE family are found in plants, animals, insects and yeast. Uncharacterized AE homologues may be present in bacteria. Animal AE proteins consist of homodimeric complexes of integral membrane proteins that vary in size from about 900 amino acyl residues to about 1250 residues. Their N-terminal hydrophilic domains may interact with cytoskeletal proteins and therefore play a cell structural role. Some of the currently characterized members of the AE family can be found in the Transporter Classification Database.

William S. Sly is an internationally known physician/scientist who, except for sabbatical years at Oxford and Stanford, spent his entire academic career in St. Louis. Following M.D. training at Saint Louis University School of Medicine, he trained in Internal Medicine at Washington University in St. Louis and in research laboratories at the NIH, in Paris, and in Madison, Wisconsin. He then joined the faculty at Washington University, where he directed the Division of Medical Genetics for 20 years. In 1984, he was recruited to St. Louis University School of Medicine and appointed Alice A. Doisy Professor and Chairman of the Edward A. Doisy Department of Biochemistry and Molecular Biology. He chaired that Department for 26 years. In February 2007, he was also named the inaugural holder of the James B. and Joan C. Peters Endowed Chair in Biochemistry and Molecular Biology. He became an Emeritus Professor in July 2014.
Send to