
A cyanide is a chemical compound that contains a C≡N functional group. This group, known as the cyano group, consists of a carbon atom triple-bonded to a nitrogen atom.
Carbon compounds are defined as chemical substances containing carbon. More compounds of carbon exist than any other chemical element except for hydrogen. Organic carbon compounds are far more numerous than inorganic carbon compounds. In general bonds of carbon with other elements are covalent bonds. Carbon is tetravalent but carbon free radicals and carbenes occur as short-lived intermediates. Ions of carbon are carbocations and carbanions are also short-lived. An important carbon property is catenation as the ability to form long carbon chains and rings.

In chemistry, a ketone is a functional group with the structure R2C=O, where R can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond). The simplest ketone is acetone (R = R' = methyl), with the formula CH3C(O)CH3. Many ketones are of great importance in biology and in industry. Examples include many sugars (ketoses), many steroids (e.g., testosterone), and the solvent acetone.

In organic chemistry, an aldehyde is an organic compound containing a functional group with the structure R−CH=O. The functional group itself can be referred to as an aldehyde but can also be classified as a formyl group. Aldehydes are common and play important roles in the technology and biological spheres.
СССР is a Russian abbreviation for the Soviet Union or Union of Soviet Socialist Republics (USSR).

Hydrazones are a class of organic compounds with the structure R1R2C=N−NH2. They are related to ketones and aldehydes by the replacement of the oxygen =O with the =N−NH2 functional group. They are formed usually by the action of hydrazine on ketones or aldehydes.
In organic chemistry, a nucleophilic addition reaction is an addition reaction where a chemical compound with an electrophilic double or triple bond reacts with a nucleophile, such that the double or triple bond is broken. Nucleophilic additions differ from electrophilic additions in that the former reactions involve the group to which atoms are added accepting electron pairs, whereas the latter reactions involve the group donating electron pairs.

Potassium cyanide is a compound with the formula KCN. This colorless crystalline salt, similar in appearance to sugar, is highly soluble in water. Most KCN is used in gold mining, organic synthesis, and electroplating. Smaller applications include jewellery for chemical gilding and buffing.
The Wolff–Kishner reduction is a reaction used in organic chemistry to convert carbonyl functionalities into methylene groups. In the context of complex molecule synthesis, it is most frequently employed to remove a carbonyl group after it has served its synthetic purpose of activating an intermediate in a preceding step. As such, there is no obvious retron for this reaction. The reaction was reported by Nikolai Kischner in 1911 and Ludwig Wolff in 1912,

Azines are a functional class of organic compounds with the connectivity RR'C=N-N=CRR'. These compounds are the product of the condensation of hydrazine with ketones and aldehydes, although in practice they are often made by alternative routes. Ketazines are azines derived from ketones, and aldazines are azines derived from aldehydes.
Umpolung or polarity inversion in organic chemistry is the chemical modification of a functional group with the aim of the reversal of polarity of that group. This modification allows secondary reactions of this functional group that would otherwise not be possible. The concept was introduced by D. Seebach and E.J. Corey. Polarity analysis during retrosynthetic analysis tells a chemist when umpolung tactics are required to synthesize a target molecule.
The Hammick reaction, named after Dalziel Hammick, is a chemical reaction in which the thermal decarboxylation of α-picolinic acids in the presence of carbonyl compounds forms 2-pyridyl-carbinols.
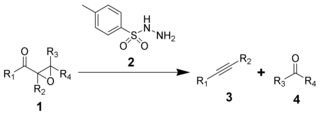
The Eschenmoser fragmentation, first published in 1967, is the chemical reaction of α,β-epoxyketones (1) with aryl sulfonylhydrazines (2) to give alkynes (3) and carbonyl compounds (4). The reaction is named after the Swiss chemist Albert Eschenmoser, who devised it in collaboration with an industrial research group of Günther Ohloff, and applied it to the production of muscone and related macrocyclic musks. The reaction is also sometimes known as the Eschenmoser–Ohloff fragmentation or the Eschenmoser–Tanabe fragmentation as Masato Tanabe independently published an article on the reaction the same year. The general formula of the fragmentation using p-toluenesulfonylhydrazide is:

Carbonyl cyanide m-chlorophenyl hydrazone is a chemical inhibitor of oxidative phosphorylation. It is a nitrile, hydrazone and protonophore. In general, CCCP causes the gradual destruction of living cells and death of the organism, although mild doses inducing partial decoupling have been shown to increase median and maximum lifespan in C. elegans models, suggesting a degree of hormesis. CCCP causes an uncoupling of the proton gradient that is established during the normal activity of electron carriers in the electron transport chain. The chemical acts essentially as an ionophore and reduces the ability of ATP synthase to function optimally. It is routinely used as an experimental uncoupling agent in cell and molecular biology, particularly in the study of mitophagy, where it was integral in discovering the role of the Parkinson's disease-associated ubiquitin ligase Parkin. Outside of its effects on mitochondria, CCCP may also disrupt lysosomal degradation during autophagy.

An uncoupling protein (UCP) is a mitochondrial inner membrane protein that is a regulated proton channel or transporter. An uncoupling protein is thus capable of dissipating the proton gradient generated by NADH-powered pumping of protons from the mitochondrial matrix to the mitochondrial intermembrane space. The energy lost in dissipating the proton gradient via UCPs is not used to do biochemical work. Instead, heat is generated. This is what links UCP to thermogenesis. However, not every type of UCPs are related to thermogenesis. Although UCP2 and UCP3 are closely related to UCP1, UCP2 and UCP3 do not affect thermoregulatory abilities of vertebrates. UCPs are positioned in the same membrane as the ATP synthase, which is also a proton channel. The two proteins thus work in parallel with one generating heat and the other generating ATP from ADP and inorganic phosphate, the last step in oxidative phosphorylation. Mitochondria respiration is coupled to ATP synthesis but is regulated by UCPs. UCPs belong to the mitochondrial carrier (SLC25) family.
A protonophore, also known as a proton translocator, is an ionophore that moves protons across lipid bilayers or other type of membranes. This would otherwise not occur as protons cations (H+) have positive charge and hydrophilic properties, making them unable to cross without a channel or transporter in the form of a protonophore. Protonophores are generally aromatic compounds with a negative charge, that are both hydrophobic and capable of distributing the negative charge over a number of atoms by π-orbitals which delocalize a proton's charge when it attaches to the molecule. Both the neutral and the charged protonophore can diffuse across the lipid bilayer by passive diffusion and simultaneously facilitate proton transport. Protonophores uncouple oxidative phosphorylation via a decrease in the membrane potential of the inner membrane of mitochondria. They stimulate mitochondria respiration and heat production. Protonophores (uncouplers) are often used in biochemistry research to help explore the bioenergetics of chemiosmotic and other membrane transport processes. It has been reported that the protonophore has antibacterial activity by perturbing bacterial proton motive force.
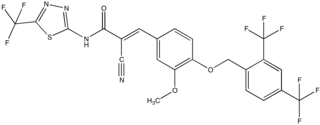
XCT-790 is a potent and selective inverse agonist ligand of the estrogen-related receptor alpha (ERRα). Independent of its inhibition of ERRα, XCT-790 is a potent mitochondrial electron transport chain uncoupler.

In organic chemistry, carbonyl reduction is the organic reduction of any carbonyl group by a reducing agent.
An uncoupler or uncoupling agent is a molecule that disrupts oxidative phosphorylation in prokaryotes and mitochondria or photophosphorylation in chloroplasts and cyanobacteria by dissociating the reactions of ATP synthesis from the electron transport chain. The result is that the cell or mitochondrion expends energy to generate a proton-motive force, but the proton-motive force is dissipated before the ATP synthase can recapture this energy and use it to make ATP. Uncouplers are capable of transporting protons through mitochondrial and lipid membranes.











