Related Research Articles

Ventricular fibrillation is an abnormal heart rhythm in which the ventricles of the heart quiver. It is due to disorganized electrical activity. Ventricular fibrillation results in cardiac arrest with loss of consciousness and no pulse. This is followed by sudden cardiac death in the absence of treatment. Ventricular fibrillation is initially found in about 10% of people with cardiac arrest.

A premature ventricular contraction (PVC) is a common event where the heartbeat is initiated by Purkinje fibers in the ventricles rather than by the sinoatrial node. PVCs may cause no symptoms or may be perceived as a "skipped beat" or felt as palpitations in the chest. PVCs do not usually pose any danger.

Propafenone, sold under the brand name Rythmol among others, is a class 1c anti-arrhythmic medication, which is used to treat illnesses associated with rapid heart beat such as atrial and ventricular arrhythmias.
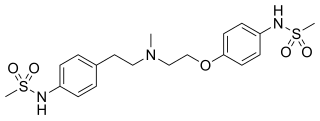
Dofetilide is a class III antiarrhythmic agent. It is marketed under the trade name Tikosyn by Pfizer, and is available in the United States in capsules containing 125, 250, and 500 µg of dofetilide. It is not available in Europe or Australia.
An inotrope or inotropic is a drug or any substance that alters the force or energy of muscular contractions. Negatively inotropic agents weaken the force of muscular contractions. Positively inotropic agents increase the strength of muscular contraction.

Flecainide is a medication used to prevent and treat abnormally fast heart rates. This includes ventricular and supraventricular tachycardias. Its use is only recommended in those with dangerous arrhythmias or when significant symptoms cannot be managed with other treatments. Its use does not decrease a person's risk of death. It is taken by mouth or injection into a vein.

Sotalol, sold under the brand name Betapace among others, is a medication used to treat and prevent abnormal heart rhythms. Evidence does not support a decreased risk of death with long term use. It is taken by mouth or given by injection into a vein.

Encainide is a class Ic antiarrhythmic agent. It is no longer used because of its frequent proarrhythmic side effects.

Lorcainide is a Class 1c antiarrhythmic agent that is used to help restore normal heart rhythm and conduction in patients with premature ventricular contractions, ventricular tachycardiac and Wolff–Parkinson–White syndrome. Lorcainide was developed by Janssen Pharmaceutica (Belgium) in 1968 under the commercial name Remivox and is designated by code numbers R-15889 or Ro 13-1042/001. It has a half-life of 8.9 +- 2.3 hrs which may be prolonged to 66 hrs in people with cardiac disease.

Dronedarone, sold under the brand name Multaq, is a medication by Sanofi-Aventis, mainly for the indication of cardiac arrhythmias. It was approved by the FDA on July 2, 2009. It was recommended as an alternative to amiodarone for the treatment of atrial fibrillation and atrial flutter in people whose hearts have either returned to normal rhythm or who undergo drug therapy or electric shock treatment i.e. direct current cardioversion (DCCV) to maintain normal rhythm. It is a class III antiarrhythmic drug. In the United States, the FDA approved label includes a claim for reducing hospitalization, but not for reducing mortality, as a reduction in mortality was not demonstrated in the clinical development program. A trial of the drug in heart failure was stopped as an interim analysis showed a possible increase in heart failure deaths, in patients with moderate to severe CHF.
Sodium channel blockers are drugs which impair the conduction of sodium ions (Na+) through sodium channels.

Michel Haïssaguerre is a French cardiologist and electrophysiologist. His investigations have been the basis for development of new markers and therapies for atrial and ventricular fibrillation.
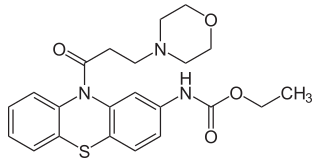
Moracizine or moricizine, sold under the trade name Ethmozine, is an antiarrhythmic of class IC. It was used for the prophylaxis and treatment of serious and life-threatening ventricular arrhythmias, but was withdrawn in 2007 for commercial reasons.

Primary ventricular fibrillation (PVF) is an unpredictable and potentially fatal arrhythmia occurring during the acute phase of a myocardial infarction leading to immediate collapse and, if left untreated, leads to sudden cardiac death within minutes. In developed countries, PVF is a leading cause of death. Worldwide, the annual number of deaths caused by PVF is comparable to the number of deaths caused by road traffic accidents. A substantial portion of these deaths could be avoided by seeking immediate medical attention when symptoms are noticed.
A wearable cardioverter defibrillator (WCD) is a non-invasive, external device for patients at risk of sudden cardiac arrest (SCA). It allows physicians time to assess their patient's arrhythmic risk and see if their ejection fraction improves before determining the next steps in patient care. It is a leased device. A summary of the device, its technology and indications was published in 2017 and reviewed by the EHRA Scientific Documents Committee.

Management of acute coronary syndrome is targeted against the effects of reduced blood flow to the affected area of the heart muscle, usually because of a blood clot in one of the coronary arteries, the vessels that supply oxygenated blood to the myocardium. This is achieved with urgent hospitalization and medical therapy, including drugs that relieve chest pain and reduce the size of the infarct, and drugs that inhibit clot formation; for a subset of patients invasive measures are also employed. Basic principles of management are the same for all types of acute coronary syndrome. However, some important aspects of treatment depend on the presence or absence of elevation of the ST segment on the electrocardiogram, which classifies cases upon presentation to either ST segment elevation myocardial infarction (STEMI) or non-ST elevation acute coronary syndrome (NST-ACS); the latter includes unstable angina and non-ST elevation myocardial infarction (NSTEMI). Treatment is generally more aggressive for STEMI patients, and reperfusion therapy is more often reserved for them. Long-term therapy is necessary for prevention of recurrent events and complications.

Celivarone is an experimental drug being tested for use in pharmacological antiarrhythmic therapy. Cardiac arrhythmia is any abnormality in the electrical activity of the heart. Arrhythmias range from mild to severe, sometimes causing symptoms like palpitations, dizziness, fainting, and even death. They can manifest as slow (bradycardia) or fast (tachycardia) heart rate, and may have a regular or irregular rhythm.

Cariporide is a selective Na+/H+ exchange inhibitor. Cariporide has been shown to actively suppress the cell death caused by oxidative stress.

HBI-3000 is an experimental drug candidate that is currently in phase II of human clinical trials as an antiarrhythmic agent. Clinical investigation will test the safety and efficacy of HBI-3000 as a treatment for both atrial and ventricular arrhythmias.
George Edward Billman is an American physiologist and professor at Ohio State University. After receiving a Ph.D from the University of Kentucky in 1980, Billman began his professional career at the University of Oklahoma. In 1984, he joined the Ohio State staff, where he became an associate professor in 1990 and a full professor in 1996.
References
- ↑ Cardiac Arrhythmia Suppression Trial (CAST) Investigators (1989). "Preliminary Report: Effect of Encainide and Flecainide on Mortality in a Randomized Trial of Arrhythmia Suppression after Myocardial Infarction". New England Journal of Medicine. 321 (6): 406–412. doi:10.1056/NEJM198908103210629. PMID 2473403.
- ↑ Echt, D. S.; Liebson, P. R.; Mitchell, L. B.; Peters, R. W.; Obias-Manno, D.; Barker, A. H.; Arensberg, D.; Baker, A.; Friedman, L.; Greene, H. L.; Huther, M. L.; Richardson, D. W. (1991). "Mortality and Morbidity in Patients Receiving Encainide, Flecainide, or Placebo". New England Journal of Medicine. 324 (12): 781–788. doi: 10.1056/NEJM199103213241201 . PMID 1900101.
- ↑ Mutschler, Ernst; Schäfer-Korting, Monika (2001). Arzneimittelwirkungen (in German) (8 ed.). Stuttgart: Wissenschaftliche Verlagsgesellschaft. p. 544. ISBN 3-8047-1763-2.
- ↑ Brooks, M. M.; Gorkin, L.; Schron, E. B.; Wiklund, I.; Campion, J.; Ledingham, R. B. (1994). "Moricizine and quality of life in the Cardiac Arrhythmia Suppression Trial II (CAST II)". Controlled Clinical Trials. 15 (6): 437–449. doi:10.1016/0197-2456(94)90002-7. PMID 7851106.
- ↑ Anderson, JL; Platia, EV; Hallstrom, A; Henthorn, RW; Buckingham, TA; Carlson, MD; Carson, PE (December 1994). "Interaction of baseline characteristics with the hazard of encainide, flecainide, and moricizine therapy in patients with myocardial infarction. A possible explanation for increased mortality in the Cardiac Arrhythmia Suppression Trial (CAST)". Circulation. 90 (6): 2843–52. doi: 10.1161/01.cir.90.6.2843 . PMID 7994829.