Related Research Articles

A COVID‑19 vaccine is a vaccine intended to provide acquired immunity against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID‑19).
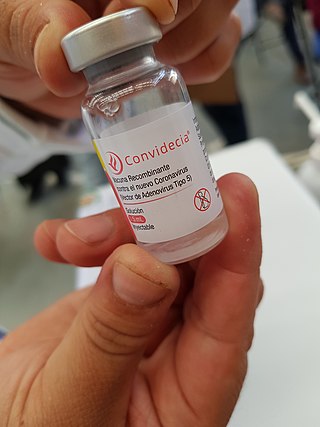
AD5-nCOV, trade-named Convidecia, is a single-dose viral vector vaccine for COVID-19 that is also used as an inhaled booster. It was developed by CanSino Biologics, with Phase III trials conducted in Argentina, Chile, Mexico, Pakistan, Russia, and Saudi Arabia with 40,000 participants.
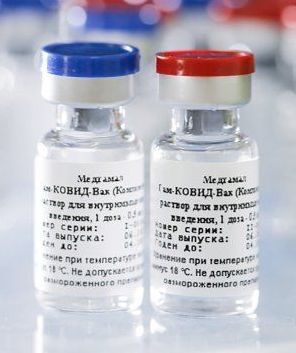
Sputnik V or Gam-COVID-Vac is an adenovirus viral vector vaccine for COVID-19 developed by the Gamaleya Research Institute of Epidemiology and Microbiology in Russia. It is the world's first registered combination vector vaccine for the prevention of COVID-19, having been registered on 11 August 2020 by the Russian Ministry of Health.

CoronaVac, also known as the Sinovac COVID-19 vaccine, is a whole inactivated virus COVID-19 vaccine developed by the Chinese company Sinovac Biotech. It was Phase III clinical trialled in Brazil, Chile, Indonesia, the Philippines, and Turkey and relies on traditional technology similar to other inactivated-virus COVID-19 vaccines, such as the Sinopharm BIBP vaccine, another Chinese vaccine, and Covaxin, an Indian vaccine. CoronaVac does not need to be frozen, and both the final product and the raw material for formulating CoronaVac can be transported refrigerated at 2–8 °C (36–46 °F), temperatures at which flu vaccines are kept.

The Sinopharm BIBP COVID-19 vaccine, also known as BBIBP-CorV, the Sinopharm COVID-19 vaccine, or BIBP vaccine, is one of two whole inactivated virus COVID-19 vaccines developed by Sinopharm's Beijing Institute of Biological Products. It completed Phase III trials in Argentina, Bahrain, Egypt, Morocco, Pakistan, Peru, and the United Arab Emirates (UAE) with over 60,000 participants. BBIBP-CorV shares similar technology with CoronaVac and Covaxin, other inactivated virus vaccines for COVID-19. Its product name is SARS-CoV-2 Vaccine, not to be confused with the similar product name of CoronaVac.

Covaxin is a whole inactivated virus-based COVID-19 vaccine developed by Bharat Biotech in collaboration with the Indian Council of Medical Research - National Institute of Virology.

The Novavax COVID-19 vaccine, sold under the brand names Nuvaxovid and Covovax, among others, is a subunit COVID-19 vaccine developed by Novavax and the Coalition for Epidemic Preparedness Innovations (CEPI). Full results from Nuvaxovid's pivotal phase III trial were published in December 2021.

ZF2001, trade-named Zifivax or ZF-UZ-VAC-2001, is an adjuvanted protein subunit COVID-19 vaccine developed by Anhui Zhifei Longcom in collaboration with the Institute of Microbiology at the Chinese Academy of Sciences. The vaccine candidate is in Phase III trials with 29,000 participants in China, Ecuador, Malaysia, Pakistan, and Uzbekistan.

The Janssen COVID‑19 vaccine, sold under the brand name Jcovden, is a COVID‑19 vaccine that was developed by Janssen Vaccines in Leiden, Netherlands, and its Belgian parent company Janssen Pharmaceuticals, a subsidiary of American company Johnson & Johnson.

India began administration of COVID-19 vaccines on 16 January 2021. As of 4 March 2023, India has administered over 2.2 billion doses overall, including first, second and precautionary (booster) doses of the currently approved vaccines. In India, 95% of the eligible population (12+) has received at least one shot, and 88% of the eligible population (12+) is fully vaccinated.

SARS-CoV-2, the virus that causes COVID-19, was isolated in late 2019. Its genetic sequence was published on 11 January 2020, triggering the urgent international response to prepare for an outbreak and hasten development of a preventive COVID-19 vaccine. Since 2020, vaccine development has been expedited via unprecedented collaboration in the multinational pharmaceutical industry and between governments. By June 2020, tens of billions of dollars were invested by corporations, governments, international health organizations, and university research groups to develop dozens of vaccine candidates and prepare for global vaccination programs to immunize against COVID‑19 infection. According to the Coalition for Epidemic Preparedness Innovations (CEPI), the geographic distribution of COVID‑19 vaccine development shows North American entities to have about 40% of the activity, compared to 30% in Asia and Australia, 26% in Europe, and a few projects in South America and Africa.
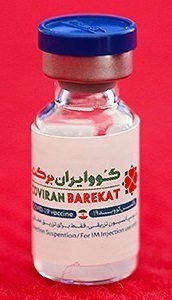
COVIran Barekat is a COVID-19 vaccine developed in Iran by Shifa Pharmed Industrial Group, a subsidiary of the Barkat Pharmaceutical Group. It is an inactivated virus-based vaccine. Iranian authorities have authorized its emergency use. This makes it the first locally developed COVID-19 vaccine to be approved for emergency use in the Middle East.

EpiVacCorona is a peptide-based vaccine against COVID-19 developed by the Russian VECTOR Center of Virology. The lack of protective effectiveness of EpiVacCorona, which is still in use in Russia, has been reported in scientific literature and in the media. The vaccine consists of three chemically synthesized peptides that are conjugated to a large carrier protein. This protein is a fusion product of a viral nucleocapsid protein and a bacterial MBP protein. A phase III clinical trial to show whether or not the vaccine can protect people against COVID-19 was launched in November 2020 with more than three thousand participants. The conclusions and results of the trial have not been made public.

The CureVac COVID-19 vaccine was a COVID-19 vaccine candidate developed by CureVac N.V. and the Coalition for Epidemic Preparedness Innovations (CEPI). The vaccine showed inadequate results in its Phase III trials with only 47% efficacy. In October 2021 CureVac abandoned further development and production plans for CVnCoV and refocused efforts on a cooperation with GlaxoSmithKline.

The COVID-19 vaccination campaign in Russia is an ongoing mass immunization campaign against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19), in response to the ongoing pandemic in the country. Russia became the first country to begin a mass COVID-19 vaccination programme on 5 December 2020, starting with primarily doctors, medical workers and teachers. In January 2021, this was extended to the entire population.

QazCovid-in, commercially known as QazVac, is a COVID-19 vaccine developed by the Research Institute for Biological Safety Problems in Kazakhstan. QazCoVac-P is a second COVID-19 vaccine developed by the Kazakh Biosafety Research Institute and in clinical trials.

The Sanofi–GSK COVID-19 vaccine sold under the brand name VidPrevtyn Beta, is a COVID-19 vaccine developed by Sanofi Pasteur and GSK.

Bangladesh began the administration of COVID-19 vaccines on 27 January 2021 while mass vaccination started on 7 February 2021.

COVID-19 vaccine clinical research uses clinical research to establish the characteristics of COVID-19 vaccines. These characteristics include efficacy, effectiveness and safety. As of November 2022, 40 vaccines are authorized by at least one national regulatory authority for public use:
References
- 1 2 3 4 5 "Russia registers world's first COVID-19 vaccine for animals". Reuters. Moscow. 31 March 2021. Retrieved 21 January 2022.
- 1 2 3 "Russia Rolls Out Covid-19 Vaccine for Animals". The Wall Street Journal. 27 May 2021. Retrieved 21 January 2022.
- 1 2 3 4 Chavda VP, Feehan J, Apostolopoulos V (10 June 2021). "A Veterinary Vaccine for SARS-CoV-2: The First COVID-19 Vaccine for Animals". Vaccines. 9 (6): 631. doi: 10.3390/vaccines9060631 . hdl:11343/287299. PMC 8228738 . PMID 34200587.
- 1 2 "From hippos to hamsters: how Covid is affecting creatures great and small". The Guardian. 11 December 2021. Retrieved 21 January 2022.
- ↑ "Russia launches vaccination of animals against COVID-19". TASS. 27 May 2021. Retrieved 21 January 2022.
- ↑ "Russia registers 'world's first' COVID-19 vaccine for animals". Al Jazeera. 31 March 2021. Retrieved 21 January 2022.
- ↑ "Russia launches production of coronavirus vaccine for animals". TASS. 30 April 2021. Retrieved 21 January 2022.
- ↑ "Covid: Russia starts vaccinating animals". BBC News. 26 May 2021. Retrieved 21 January 2022.