Catalysis is the increase in rate of a chemical reaction due to an added substance known as a catalyst. Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quickly, very small amounts of catalyst often suffice; mixing, surface area, and temperature are important factors in reaction rate. Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product, in the process of regenerating the catalyst.

Surface science is the study of physical and chemical phenomena that occur at the interface of two phases, including solid–liquid interfaces, solid–gas interfaces, solid–vacuum interfaces, and liquid–gas interfaces. It includes the fields of surface chemistry and surface physics. Some related practical applications are classed as surface engineering. The science encompasses concepts such as heterogeneous catalysis, semiconductor device fabrication, fuel cells, self-assembled monolayers, and adhesives. Surface science is closely related to interface and colloid science. Interfacial chemistry and physics are common subjects for both. The methods are different. In addition, interface and colloid science studies macroscopic phenomena that occur in heterogeneous systems due to peculiarities of interfaces.
Chemisorption is a kind of adsorption which involves a chemical reaction between the surface and the adsorbate. New chemical bonds are generated at the adsorbent surface. Examples include macroscopic phenomena that can be very obvious, like corrosion, and subtler effects associated with heterogeneous catalysis, where the catalyst and reactants are in different phases. The strong interaction between the adsorbate and the substrate surface creates new types of electronic bonds.

Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the adsorbate on the surface of the adsorbent. This process differs from absorption, in which a fluid is dissolved by or permeates a liquid or solid. While adsorption does often precede absorption, which involves the transfer of the absorbate into the volume of the absorbent material, alternatively, adsorption is distinctly a surface phenomenon, wherein the adsorbate does not penetrate through the material surface and into the bulk of the adsorbent. The term sorption encompasses both adsorption and absorption, and desorption is the reverse of sorption.

Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically constitutes the addition of pairs of hydrogen atoms to a molecule, often an alkene. Catalysts are required for the reaction to be usable; non-catalytic hydrogenation takes place only at very high temperatures. Hydrogenation reduces double and triple bonds in hydrocarbons.
In chemistry, dehydrogenation is a chemical reaction that involves the removal of hydrogen, usually from an organic molecule. It is the reverse of hydrogenation. Dehydrogenation is important, both as a useful reaction and a serious problem. At its simplest, it's a useful way of converting alkanes, which are relatively inert and thus low-valued, to olefins, which are reactive and thus more valuable. Alkenes are precursors to aldehydes, alcohols, polymers, and aromatics. As a problematic reaction, the fouling and inactivation of many catalysts arises via coking, which is the dehydrogenative polymerization of organic substrates.

Heterogeneous catalysis is catalysis where the phase of catalysts differs from that of the reagents or products. The process contrasts with homogeneous catalysis where the reagents, products and catalyst exist in the same phase. Phase distinguishes between not only solid, liquid, and gas components, but also immiscible mixtures, or anywhere an interface is present.
The water–gas shift reaction (WGSR) describes the reaction of carbon monoxide and water vapor to form carbon dioxide and hydrogen:

In chemistry, a catalytic cycle is a multistep reaction mechanism that involves a catalyst. The catalytic cycle is the main method for describing the role of catalysts in biochemistry, organometallic chemistry, bioinorganic chemistry, materials science, etc.
In chemistry, a phase-transfer catalyst or PTC is a catalyst that facilitates the transition of a reactant from one phase into another phase where reaction occurs. Phase-transfer catalysis is a special form of catalysis and can act through homogeneous catalysis or heterogeneous catalysis methods depending on the catalyst used. Ionic reactants are often soluble in an aqueous phase but insoluble in an organic phase in the absence of the phase-transfer catalyst. The catalyst functions like a detergent for solubilizing the salts into the organic phase. Phase-transfer catalysis refers to the acceleration of the reaction upon the addition of the phase-transfer catalyst.
Nanomaterial-based catalysts are usually heterogeneous catalysts broken up into metal nanoparticles in order to enhance the catalytic process. Metal nanoparticles have high surface area, which can increase catalytic activity. Nanoparticle catalysts can be easily separated and recycled. They are typically used under mild conditions to prevent decomposition of the nanoparticles.
Reactive flash volatilization (RFV) is a chemical process that rapidly converts nonvolatile solids and liquids to volatile compounds by thermal decomposition for integration with catalytic chemistries.

An electrocatalyst is a catalyst that participates in electrochemical reactions. Electrocatalysts are a specific form of catalysts that function at electrode surfaces or, most commonly, may be the electrode surface itself. An electrocatalyst can be heterogeneous such as a platinized electrode. Homogeneous electrocatalysts, which are soluble, assist in transferring electrons between the electrode and reactants, and/or facilitate an intermediate chemical transformation described by an overall half reaction. Major challenges in electrocatalysts focus on fuel cells.
Organogold chemistry is the study of compounds containing gold–carbon bonds. They are studied in academic research, but have not received widespread use otherwise. The dominant oxidation states for organogold compounds are I with coordination number 2 and a linear molecular geometry and III with CN = 4 and a square planar molecular geometry.
The first time a catalyst was used in the industry was in 1746 by J. Roebuck in the manufacture of lead chamber sulfuric acid. Since then catalysts have been in use in a large portion of the chemical industry. In the start only pure components were used as catalysts, but after the year 1900 multicomponent catalysts were studied and are now commonly used in the industry.
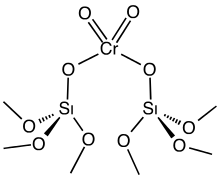
Carbon nanotube supported catalyst is a novel supported catalyst, using carbon nanotubes as the support instead of the conventional alumina or silicon support. The exceptional physical properties of carbon nanotubes (CNTs) such as large specific surface areas, excellent electron conductivity incorporated with the good chemical inertness, and relatively high oxidation stability makes it a promising support material for heterogeneous catalysis.

Cyclohexene oxide is a cycloaliphatic epoxide. It can react in cationic polymerization to poly(cyclohexene oxide). As cyclohexene is monovalent, poly(cyclohexene oxide) is a thermoplastic.
The electrochemical promotion of catalysis (EPOC) effect in the realm of chemistry refers to the pronounced enhancement of catalytic reactions or significant changes in the catalytic properties of a conductive catalyst in the presence of electrical currents or interfacial potentials. Also known as Non-faradaic electrochemical modification of catalytic activity (the NEMCA effect), it can increase in catalytic activity (up to 90-fold) and selectivity of a gas exposed electrode on a solid electrolyte cell upon application of a potential. This phenomenon is well documented and has been observed on various surfaces (Ni, Au, Pt, Pd, IrO2, RuO2) supported by O2−, Na+ and proton conducting solid electrolytes.

Heterogeneous gold catalysis refers to the use of elemental gold as a heterogeneous catalyst. As in most heterogeneous catalysis, the metal is typically supported on metal oxide. Furthermore, as seen in other heterogeneous catalysts, activity increases with a decreasing diameter of supported gold clusters. Several industrially relevant processes are also observed such as H2 activation, Water-gas shift reaction, and hydrogenation. One or two gold-catalyzed reactions may have been commercialized.
Heterogeneous metal catalyzed cross-coupling is a subset of metal catalyzed cross-coupling in which a heterogeneous metal catalyst is employed. Generally heterogeneous cross-coupling catalysts consist of a metal dispersed on an inorganic surface or bound to a polymeric support with ligands. Heterogeneous catalysts provide potential benefits over homogeneous catalysts in chemical processes in which cross-coupling is commonly employed—particularly in the fine chemical industry—including recyclability and lower metal contamination of reaction products. However, for cross-coupling reactions, heterogeneous metal catalysts can suffer from pitfalls such as poor turnover and poor substrate scope, which have limited their utility in cross-coupling reactions to date relative to homogeneous catalysts. Heterogeneous metal catalyzed cross-couplings, as with homogeneous metal catalyzed ones, most commonly use Pd as the cross-coupling metal.