Related Research Articles

Kidney dialysis is the process of removing excess water, solutes, and toxins from the blood in people whose kidneys can no longer perform these functions naturally. This is referred to as renal replacement therapy. The first successful dialysis was performed in 1943.

A central venous catheter (CVC), also known as a central line (c-line), central venous line, or central venous access catheter, is a catheter placed into a large vein. It is a form of venous access. Placement of larger catheters in more centrally located veins is often needed in critically ill patients, or in those requiring prolonged intravenous therapies, for more reliable vascular access. These catheters are commonly placed in veins in the neck, chest, groin, or through veins in the arms.
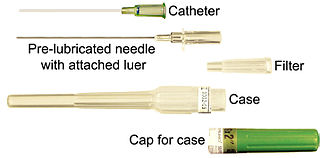
In medicine, a catheter (/ˈkæθətər/) is a thin tube made from medical grade materials serving a broad range of functions. Catheters are medical devices that can be inserted in the body to treat diseases or perform a surgical procedure. Catheters are manufactured for specific applications, such as cardiovascular, urological, gastrointestinal, neurovascular and ophthalmic procedures. The process of inserting a catheter is called catheterization.
Bloodstream infections (BSIs) are infections of blood caused by blood-borne pathogens. Blood is normally a sterile environment, so the detection of microbes in the blood is always abnormal. A bloodstream infection is different from sepsis, which is characterized by severe inflammatory or immune responses of the host organism to pathogens.

Hemodialysis, also spelled haemodialysis, or simply dialysis, is a process of filtering the blood of a person whose kidneys are not working normally. This type of dialysis achieves the extracorporeal removal of waste products such as creatinine and urea and free water from the blood when the kidneys are in a state of kidney failure. Hemodialysis is one of three renal replacement therapies. An alternative method for extracorporeal separation of blood components such as plasma or cells is apheresis.
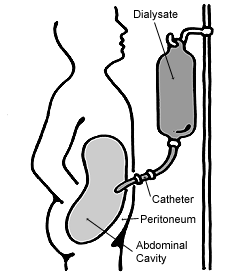
Peritoneal dialysis (PD) is a type of dialysis that uses the peritoneum in a person's abdomen as the membrane through which fluid and dissolved substances are exchanged with the blood. It is used to remove excess fluid, correct electrolyte problems, and remove toxins in those with kidney failure. Peritoneal dialysis has better outcomes than hemodialysis during the first couple of years. Other benefits include greater flexibility and better tolerability in those with significant heart disease.
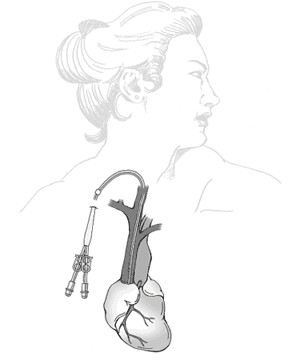
A dialysis catheter is a catheter used for exchanging blood to and from a hemodialysis machine and a patient.

A peripherally inserted central catheter, less commonly called a percutaneous indwelling central catheter, is a form of intravenous access that can be used for a prolonged period of time or for administration of substances that should not be done peripherally. It is a catheter that enters the body through the skin (percutaneously) at a peripheral site, extends to the superior vena cava, and stays in place for days, weeks or even months.

Home hemodialysis (HHD) is the provision of hemodialysis to purify the blood of a person whose kidneys are not working normally, in their own home. One advantage to doing dialysis at home is that it can be done more frequently and slowly, which reduces the "washed out" feeling and other symptoms caused by rapid ultrafiltration, and it can often be done at night, while the person is sleeping.

Hemofiltration, also haemofiltration, is a renal replacement therapy which is used in the intensive care setting. It is usually used to treat acute kidney injury (AKI), but may be of benefit in multiple organ dysfunction syndrome or sepsis. During hemofiltration, a patient's blood is passed through a set of tubing via a machine to a semipermeable membrane where waste products and water are removed by convection. Replacement fluid is added and the blood is returned to the patient.
Renal replacement therapy (RRT) is therapy that replaces the normal blood-filtering function of the kidneys. It is used when the kidneys are not working well, which is called kidney failure and includes acute kidney injury and chronic kidney disease. Renal replacement therapy includes dialysis, hemofiltration, and hemodiafiltration, which are various ways of filtration of blood with or without machines. Renal replacement therapy also includes kidney transplantation, which is the ultimate form of replacement in that the old kidney is replaced by a donor kidney.

Temocillin is a β-lactamase-resistant penicillin introduced by Beecham, marketed by Eumedica Pharmaceuticals as Negaban. It is used primarily for the treatment of multiple drug-resistant, Gram-negative bacteria.
It is a 6-methoxy penicillin; it is also a carboxypenicillin.
Vascular access refers to a rapid, direct method of introducing or removing devices or chemicals from the bloodstream. In hemodialysis, vascular access is used to remove the patient's blood so that it can be filtered through the dialyzer. Three primary methods are used to gain access to the blood: an intravenous catheter, an arteriovenous fistula (AV) or a synthetic graft. In the latter two, needles are used to puncture the graft or fistula each time dialysis is performed.
Haemodialysis-associated amyloidosis is a form of systemic amyloidosis associated with chronic kidney failure. Amyloidosis is the accumulation of misfolded protein fibers in the body that can be associated with many chronic illnesses. Even though amyloidosis is common in chronic kidney disease (CKD) patients receiving chronic regular dialysis, it has also been reported in a patient with chronic kidney failure but who never received dialysis.
Quinton catheters are non-tunneled central line catheters, which are often used for acute access for hemodialysis or infusion of medicine when peripheral IV access is not possible. They can also be used to infuse liquids which cause peripheral blood vessel irritation, directly into the vena cavae where they are immediately diluted.

Taurolidine is an antimicrobial that is used to prevent infections in catheters. Side effects and the induction of bacterial resistance is uncommon. It is also being studied as a treatment for cancer.
Ischemic monomelic neuropathy(IMN) is an uncommon vascular access complication in hemodialysis patients that manifests as multiple mononeuropathies without clinical ischemia. Ischemic monomelic neuropathy is most likely to affect patients who have had brachiocephalic vascular grafts, and it is characterized by symptoms of acute pain, numbness, and paresthesia in addition to motor weakness. The term "ischemic monomelic neuropathy" was first used in 1983 by Wilbourn, despite the fact that Bolton et al. had originally reported on it in 1979.
Kerry L. LaPlante is an American pharmacist, academic and researcher. She is the Dean at the University of Rhode Island College of Pharmacy. She is a Professor of Pharmacy and former department Chair of the Department of Pharmacy Practice at the University of Rhode Island, an Adjunct Professor of Medicine at Brown University, an Infectious Diseases Pharmacotherapy Specialist, and the Director of the Rhode Island Infectious Diseases Fellowship and Research Programs at the Veterans Affairs Medical Center in Providence, Rhode Island.
Stanley Shaldon was a British nephrologist who pioneered several techniques in haemodialysis, including venous access, reuse of dialysis machines, and home haemodialysis.
Taurolidine/heparin, sold under the brand name Defencath, is a fixed-dose combination catheter lock solution used for central venous catheter instillation. It contains taurolidine, a thiadiazinane antimicrobial; and heparin, an anti-coagulant. Its use is limited to people with kidney failure receiving chronic hemodialysis through a central venous catheter.
References
- ↑ Neutrolin Product Brochure Archived 2014-02-02 at the Wayback Machine .
- ↑ Solomon, L.R.; et al. (June 2010). "A Randomized Double-Blind Controlled Trial of Taurolidine-Citrate Catheter Locks for the Prevention of Bacteremia in Patients Treated with Hemodialysis". American Journal of Kidney Diseases. 55 (6): 1060–1068. doi:10.1053/j.ajkd.2009.11.025. PMID 20207458.
- ↑ Koldehoff M.; Zakrzewski J.L. (June 2004). "Taurolidine is effective in the treatment of central venous catheter-related bloodstream infections in cancer patients". International Journal of Antimicrobial Agents. 24 (5): 491–495. doi: 10.1016/j.ijantimicag.2004.06.006 . PMID 15519483.
- ↑ Simon A.; Ammann R.A.; Wiszniewsky G.; Bode U.; Fleischhack G. & Besuden M.M. (June 2008). "Taurolidine-citrate lock solution significantly reduces CVAD-associated grampositive infections in pediatric cancer patients". BMC Infectious Diseases. 8: 102. doi: 10.1186/1471-2334-8-102 . PMC 2515312 . PMID 18664278.
- ↑ Betjes M.G.H. & van Agteren M. (February 2004). "Prevention of dialysis catheter-related sepsis with a citrate-taurolidine-containing lock solution". Nephrology Dialysis Transplantation. 19 (6): 1546–1551. doi: 10.1093/ndt/gfh014 . PMID 14993498.
- ↑ Handrup M.M., Moller, J.K., and Schroder, H. (February 2013). "Central Venous Catheters and Catheter Locks in Children With Cancer: A Prospective Randomized Trial of Taurolidine Versus Heparin". Pediatric Blood & Cancer. 60 (8): 1292–1298. doi: 10.1002/pbc.24482 . PMID 23417891. S2CID 39838675.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Allon, Michael (2003-06-15). "Prophylaxis against dialysis catheter-related bacteremia with a novel antimicrobial lock solution". Clinical Infectious Diseases. 36 (12): 1539–1544. doi: 10.1086/375234 . ISSN 1537-6591. PMID 12802753.
- ↑ Karaaslan, H.; Peyronnet, P.; Benevent, D.; Lagarde, C.; Rince, M.; Leroux-Robert, C. (2001). "Risk of heparin lock-related bleeding when using an indwelling venous catheter in haemodialysis". Nephrology Dialysis Transplantation. 16 (10): 2072–4. doi: 10.1093/ndt/16.10.2072 . PMID 11572899.
- ↑ Agharazii, Mohsen; Plamondon, Isabelle; Lebel, Marcel; Douville, Pierre; Desmeules, Simon (2005). "Estimation of heparin leak into the systemic circulation after central venous catheter heparin lock". Nephrology Dialysis Transplantation. 20 (6): 1238–1240. doi: 10.1093/ndt/gfh841 . PMID 15855206.
- ↑ Vanholder, R.; Canaud, B.; Fluck, R.; Jadoul, M.; Labriola, L.; Marti-Monros, A.; Tordoir, J.; Van Biesen, W. (2010). "Diagnosis, prevention and treatment of haemodialysis catheter-related bloodstream infections (CRBSI): a position statement of European Renal Best Practice (ERBP)". NDT Plus. 3 (3): 234–246. doi:10.1093/ndtplus/sfq041. PMC 6371390 . PMID 30792802.
- ↑ Ash, S. R.; Mankus, R. A.; Sutton, J. M.; Criswell, R. E.; Crull, C. C.; Velasquez, K. A.; Smeltzer, B. D.; Ing, T. S. (2000). "Concentrated Sodium Citrate (23%) for catheter lock" (PDF). Hemodialysis International. 4 (1): 22–31. doi:10.1111/hdi.2000.4.1.22. PMID 28455911. S2CID 914726.
- ↑ Weijmer, M. C.; Debets-Ossenkopp, Y. J.; Van De Vondervoort, F. J.; Ter Wee, P. M. (2002). "Superior antimicrobial activity of trisodium citrate over heparin for catheter locking". Nephrology Dialysis Transplantation. 17 (12): 2189–2195. doi: 10.1093/ndt/17.12.2189 . PMID 12454232.
- ↑ "FDA approves new drug under special pathway for patients receiving hemodialysis". U.S. Food and Drug Administration (FDA). 15 November 2023. Archived from the original on 12 December 2023. Retrieved 16 November 2023.
 This article incorporates text from this source, which is in the public domain .
This article incorporates text from this source, which is in the public domain . - ↑ "CorMedix Inc. Announces FDA Approval of Defencath to Reduce the Incidence of Catheter-Related Bloodstream Infections in Adult Hemodialysis Patients" (Press release). Cormedix Inc. 15 November 2023. Retrieved 16 November 2023– via GlobeNewswire.