
The citric acid cycle (CAC) – also known as the TCA cycle or the Krebs cycle – is a series of chemical reactions to release stored energy through the oxidation of acetyl-CoA derived from carbohydrates, fats, and proteins. The Krebs cycle is used by organisms that respire to generate energy, either by anaerobic respiration or aerobic respiration. In addition, the cycle provides precursors of certain amino acids, as well as the reducing agent NADH, that are used in numerous other reactions. Its central importance to many biochemical pathways suggests that it was one of the earliest components of metabolism and may have originated abiogenically. Even though it is branded as a 'cycle', it is not necessary for metabolites to follow only one specific route; at least three alternative segments of the citric acid cycle have been recognized.

Glycolysis is the metabolic pathway that converts glucose C6H12O6, into pyruvic acid, CH3COCOOH. The free energy released in this process is used to form the high-energy molecules adenosine triphosphate (ATP) and reduced nicotinamide adenine dinucleotide (NADH). Glycolysis is a sequence of ten reactions catalyzed by enzymes.

Adenosine diphosphate (ADP), also known as adenosine pyrophosphate (APP), is an important organic compound in metabolism and is essential to the flow of energy in living cells. ADP consists of three important structural components: a sugar backbone attached to adenine and two phosphate groups bonded to the 5 carbon atom of ribose. The diphosphate group of ADP is attached to the 5’ carbon of the sugar backbone, while the adenine attaches to the 1’ carbon.
An anaerobic organism or anaerobe is any organism that does not require molecular oxygen for growth. It may react negatively or even die if free oxygen is present. In contrast, an aerobic organism (aerobe) is an organism that requires an oxygenated environment. Anaerobes may be unicellular or multicellular. Most fungi are obligate aerobes, requiring oxygen to survive. However, some species, such as the Chytridiomycota that reside in the rumen of cattle, are obligate anaerobes; for these species, anaerobic respiration is used because oxygen will disrupt their metabolism or kill them. Deep waters of the ocean are a common anoxic environment.

Cellular respiration is a set of metabolic reactions and processes that take place in the cells of organisms to convert chemical energy from oxygen molecules or nutrients into adenosine triphosphate (ATP), and then release waste products. The reactions involved in respiration are catabolic reactions, which break large molecules into smaller ones, releasing energy because weak high-energy bonds, in particular in molecular oxygen, are replaced by stronger bonds in the products. Respiration is one of the key ways a cell releases chemical energy to fuel cellular activity. The overall reaction occurs in a series of biochemical steps, some of which are redox reactions. Although cellular respiration is technically a combustion reaction, it clearly does not resemble one when it occurs in a living cell because of the slow, controlled release of energy from the series of reactions.

Lactic acid is an organic acid. It has a molecular formula CH3CH(OH)COOH. It is white in the solid state and it is miscible with water. When in the dissolved state, it forms a colorless solution. Production includes both artificial synthesis as well as natural sources. Lactic acid is an alpha-hydroxy acid (AHA) due to the presence of a hydroxyl group adjacent to the carboxyl group. It is used as a synthetic intermediate in many organic synthesis industries and in various biochemical industries. The conjugate base of lactic acid is called lactate.
Anaerobic glycolysis is the transformation of glucose to lactate when limited amounts of oxygen (O2) are available. Anaerobic glycolysis is only an effective means of energy production during short, intense exercise, providing energy for a period ranging from 10 seconds to 2 minutes. This is much faster than aerobic metabolism. The anaerobic glycolysis (lactic acid) system is dominant from about 10–30 seconds during a maximal effort. It replenishes very quickly over this period and produces 2 ATP molecules per glucose molecule, or about 5% of glucose's energy potential (38 ATP molecules). The speed at which ATP is produced is about 100 times that of oxidative phosphorylation.
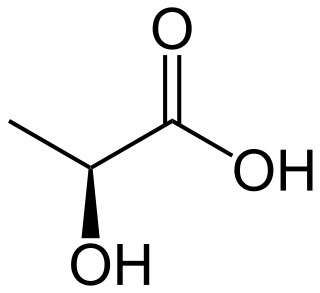
Lactic acid fermentation is a metabolic process by which glucose or other six-carbon sugars are converted into cellular energy and the metabolite lactate, which is lactic acid in solution. It is an anaerobic fermentation reaction that occurs in some bacteria and animal cells, such as muscle cells.
Digestion is the breakdown of carbohydrates to yield an energy rich compound called ATP. The production of ATP is achieved through the oxidation of glucose molecules. In oxidation, the electrons are stripped from a glucose molecule to reduce NAD+ and FAD. NAD+ and FAD possess a high energy potential to drive the production of ATP in the electron transport chain. ATP production occurs in the mitochondria of the cell. There are two methods of producing ATP: aerobic and anaerobic. In aerobic respiration, oxygen is required. Oxygen as a high-energy molecule increases ATP production from 4 ATP molecules to about 30 ATP molecules. In anaerobic respiration, oxygen is not required. When oxygen is absent, the generation of ATP continues through fermentation. There are two types of fermentation: alcohol fermentation and lactic acid fermentation.
Carbohydrate metabolism is the whole of the biochemical processes responsible for the metabolic formation, breakdown, and interconversion of carbohydrates in living organisms.
Anaerobic respiration is respiration using electron acceptors other than molecular oxygen (O2). Although oxygen is not the final electron acceptor, the process still uses a respiratory electron transport chain.

Obligate anaerobes are microorganisms killed by normal atmospheric concentrations of oxygen (20.95% O2). Oxygen tolerance varies between species, with some species capable of surviving in up to 8% oxygen, while others lose viability in environments with an oxygen concentration greater than 0.5%.

Anaerobic exercise is a type of exercise that breaks down glucose in the body without using oxygen; anaerobic means "without oxygen". In practical terms, this means that anaerobic exercise is more intense, but shorter in duration than aerobic exercise.
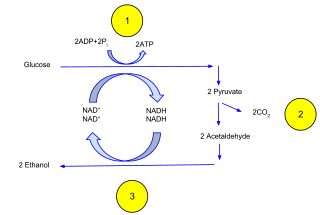
Ethanol fermentation, also called alcoholic fermentation, is a biological process which converts sugars such as glucose, fructose, and sucrose into cellular energy, producing ethanol and carbon dioxide as by-products. Because yeasts perform this conversion in the absence of oxygen, alcoholic fermentation is considered an anaerobic process. It also takes place in some species of fish where it provides energy when oxygen is scarce.

The Cori cycle, named after its discoverers, Carl Ferdinand Cori and Gerty Cori, is a metabolic pathway in which lactate produced by anaerobic glycolysis in muscles is transported to the liver and converted to glucose, which then returns to the muscles and is cyclically metabolized back to lactate.
In oncology, the Warburg effect is a form of modified cellular metabolism found in cancer cells, which tend to favor a specialized fermentation over the aerobic respiration pathway that most other cells of the body prefer. This observation was first published by Otto Heinrich Warburg, who was awarded the 1931 Nobel Prize in Physiology for his "discovery of the nature and mode of action of the respiratory enzyme".

Fermentation is a metabolic process that produces chemical changes in organic substrates through the action of enzymes. In biochemistry, it is narrowly defined as the extraction of energy from carbohydrates in the absence of oxygen. In food production, it may more broadly refer to any process in which the activity of microorganisms brings about a desirable change to a foodstuff or beverage. The science of fermentation is known as zymology.
The Pasteur effect is an inhibiting effect of oxygen on the fermentation process. It is a sudden change from anaerobic to aerobic process.
Bioenergetic systems are metabolic processes that relate to the flow of energy in living organisms. Those processes convert energy into adenosine triphosphate (ATP), which is the form suitable for muscular activity. There are two main forms of synthesis of ATP: aerobic, which uses oxygen from the bloodstream, and anaerobic, which does not. Bioenergetics is the field of biology that studies bioenergetic systems.
Aerobic fermentation or aerobic glycolysis is a metabolic process by which cells metabolize sugars via fermentation in the presence of oxygen and occurs through the repression of normal respiratory metabolism. It is referred to as the Crabtree effect in yeast. and is part of the Warburg effect in tumor cells. While aerobic fermentation does not produce adenosine triphosphate (ATP) in high yield, it allows proliferating cells to convert nutrients such as glucose and glutamine more efficiently into biomass by avoiding unnecessary catabolic oxidation of such nutrients into carbon dioxide, preserving carbon-carbon bonds and promoting anabolism.











