The following outline is provided as an overview of and topical guide to chemistry:
Physical science is a branch of natural science that studies non-living systems, in contrast to life science. It in turn has many branches, each referred to as a "physical science", together is called the "physical sciences".
An electrolyte is a medium containing ions that are electrically conductive through the movement of those ions, but not conducting electrons. This includes most soluble salts, acids, and bases dissolved in a polar solvent, such as water. Upon dissolving, the substance separates into cations and anions, which disperse uniformly throughout the solvent. Solid-state electrolytes also exist. In medicine and sometimes in chemistry, the term electrolyte refers to the substance that is dissolved.

The Council of Scientific and Industrial Research is a research and development (R&D) organisation in India to promote scientific, industrial and economic growth. Headquartered in New Delhi, it was established as an autonomous body in 1942 under the aegis of the Department of Scientific and Industrial Research (DSIR), Ministry of Science and Technology, Government of India. CSIR is among the largest publicly funded R&D organisations in the world. CSIR has pioneered sustained contribution to science and technology (S&T) human resource development in India.

Electrophoretic deposition (EPD), is a term for a broad range of industrial processes which includes electrocoating, cathodic electrodeposition, anodic electrodeposition, and electrophoretic coating, or electrophoretic painting. A characteristic feature of this process is that colloidal particles suspended in a liquid medium migrate under the influence of an electric field (electrophoresis) and are deposited onto an electrode. All colloidal particles that can be used to form stable suspensions and that can carry a charge can be used in electrophoretic deposition. This includes materials such as polymers, pigments, dyes, ceramics and metals.
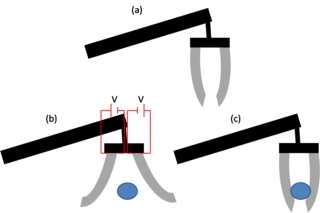
An electroactive polymer (EAP) is a polymer that exhibits a change in size or shape when stimulated by an electric field. The most common applications of this type of material are in actuators and sensors. A typical characteristic property of an EAP is that they will undergo a large amount of deformation while sustaining large forces.

Ion exchange is a reversible interchange of one species of ion present in an insoluble solid with another of like charge present in a solution surrounding the solid. Ion exchange is used in softening or demineralizing of water, purification of chemicals, and separation of substances.

The Bulgarian Academy of Sciences is the National Academy of Bulgaria, established in 1869.

Electrodialysis (ED) is used to transport salt ions from one solution through ion-exchange membranes to another solution under the influence of an applied electric potential difference. This is done in a configuration called an electrodialysis cell. The cell consists of a feed (dilute) compartment and a concentrate (brine) compartment formed by an anion exchange membrane and a cation exchange membrane placed between two electrodes. In almost all practical electrodialysis processes, multiple electrodialysis cells are arranged into a configuration called an electrodialysis stack, with alternating anion and cation-exchange membranes forming the multiple electrodialysis cells. Electrodialysis processes are different from distillation techniques and other membrane based processes in that dissolved species are moved away from the feed stream, whereas other processes move away the water from the remaining substances. Because the quantity of dissolved species in the feed stream is far less than that of the fluid, electrodialysis offers the practical advantage of much higher feed recovery in many applications.
The National Academy of Sciences Award in Chemical Sciences is awarded for innovative research in the chemical sciences that in the broadest sense contributes to a better understanding of the natural sciences and to the benefit of humanity.

Osmotic power, salinity gradient power or blue energy is the energy available from the difference in the salt concentration between seawater and river water. Two practical methods for this are reverse electrodialysis (RED) and pressure retarded osmosis (PRO). Both processes rely on osmosis with membranes. The key waste product is brackish water. This byproduct is the result of natural forces that are being harnessed: the flow of fresh water into seas that are made up of salt water.

The National Institute for Interdisciplinary Science and Technology is a constituent laboratory of CSIR, India, engaged in research and development activities in the field of agroprocessing and technology, microbial processes and technology, chemical sciences and technology, material sciences and technology and process engineering and environmental technology. Around approximately 80 scientists and 300 research fellows are working in various scientific disciplines in this institute. The programmes have a blend of basic research, technology development and commercialization; have specific thrusts on frontier areas of research, National Mission Projects, regional resource-based activities and R & D - Industry - Academia linkages. The laboratory has excellent collaborative programmes with major National & International agencies too. the present director of the institute is Dr.C. Anandharamakrishnan.
The Willard Gibbs Award, presented by the Chicago Section of the American Chemical Society, was established in 1910 by William A. Converse (1862–1940), a former Chairman and Secretary of the Chicago Section of the society and named for Professor Josiah Willard Gibbs (1839–1903) of Yale University. Gibbs, whose formulation of the Phase Rule founded a new science, is considered by many to be the only American-born scientist whose discoveries are as fundamental in nature as those of Newton and Galileo.
Carl Shipp "Speed" Marvel was an American chemist who specialized in polymer chemistry. He made important contributions to U.S. synthetic rubber program during World War II, and later worked at developing polybenzimidazoles, temperature-resistant polymers that are used in the aerospace industry, in fire-fighting equipment, and as a replacement for asbestos. He has been described as "one of the world's outstanding organic chemists" and received numerous awards, including the 1956 Priestley Medal and the 1986 National Medal of Science, presented by President Ronald Reagan.
Ayyappanpillai Ajayaghosh is a research scientist/academician in the domain of interdisciplinary chemistry, and the former Director of the National Institute for Interdisciplinary Science and Technology. He is known for his studies on supramolecular assemblies, organogels, photoresponsive materials, chemosensory and security materials systems and is an elected fellow of all the three major Indian science academies viz. the National Academy of Sciences, India, Indian National Science Academy and the Indian Academy of Sciences as well as The World Academy of Sciences. The Council of Scientific and Industrial Research, the apex agency of the Government of India for scientific research, awarded him the Shanti Swarup Bhatnagar Prize for Science and Technology, one of the highest Indian science awards for his contributions to Chemical Sciences in 2007. He is the first chemist to receive the Infosys Science Prize for physical sciences, awarded by the Infosys Science Foundation. He received the TWAS Prize of The World Academy of Sciences in 2013 and the Goyal prize in 2019.
Paramasivam Natarajan (1940–2016) was an Indian photochemist, the INSA Senior Scientist at the National Centre for Ultrafast Process of the University of Madras and the director of Central Salt and Marine Chemicals Research Institute (CSMCRI) of the Council of Scientific and Industrial Research. He was known for his research on photochemistry of co-ordination compounds and macromolecular dye coatings for stabilization of electrodes. He was an elected fellow of the Indian National Science Academy, International Union of Pure and Applied Chemistry (IUPAC) and the Indian Academy of Sciences. The Council of Scientific and Industrial Research, the apex agency of the Government of India for scientific research, awarded him the Shanti Swarup Bhatnagar Prize for Science and Technology, one of the highest Indian science awards, in 1984, for his contributions to chemical sciences.

Dumbala Srinivasa Reddy is currently Director CSIR-IICT Indian Institute of Chemical Technology Hyderabad India, he has additional charge of CSIR-IIIM CSIR-Institute of Integrative Medicine at Jammu and CSIR-CDRI CSIR-Drug Research Institute at Lucknow, India.
Pradyut Ghosh is an Indian inorganic chemist and a professor at the Indian Association for the Cultivation of Science. He is known for his studies on chemical sensing of anions, interlocked molecules and self-assembly. He is a recipient of the Swarnajayanthi Fellowship of the Department of Science and Technology and the Bronze Medal of the Chemical Research Society of India. The Council of Scientific and Industrial Research, the apex agency of the Government of India for scientific research, awarded him the Shanti Swarup Bhatnagar Prize for Science and Technology, one of the highest Indian science awards, in 2015, for his contributions to chemical sciences.
Bhaskar Dattatraya Kulkarni, popularly known as B. D. among his friends and colleagues, was an Indian chemical reaction engineer and a Distinguished Scientist of Chemical Engineering and Process Development at the National Chemical Laboratory, Pune. An INSA Senior Scientist and a J. C. Bose fellow, he was known for his work on fluidized bed reactors and chemical reactors. He is an elected fellow of the Indian Academy of Sciences, Indian National Science Academy, The World Academy of Sciences and the Indian National Academy of Engineering. The Council of Scientific and Industrial Research, the apex agency of the Government of India for scientific research, awarded him the Shanti Swarup Bhatnagar Prize for Science and Technology, one of the highest Indian science awards for his contributions to Engineering Sciences in 1988.

Alexander Butlerov Chemistry Institute — structural unit of Kazan Federal University, carries out research, development and academic activity in the area of basic and applied chemistry.









