Related Research Articles
The actinide or actinoid series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium. The actinide series derives its name from the first element in the series, actinium. The informal chemical symbol An is used in general discussions of actinide chemistry to refer to any actinide.

Monazite is a primarily reddish-brown phosphate mineral that contains rare-earth elements. Due to variability in composition, monazite is considered a group of minerals. The most common species of the group is monazite-(Ce), that is, the cerium-dominant member of the group. It occurs usually in small isolated crystals. It has a hardness of 5.0 to 5.5 on the Mohs scale of mineral hardness and is relatively dense, about 4.6 to 5.7 g/cm3. There are five different most common species of monazite, depending on the relative amounts of the rare earth elements in the mineral:
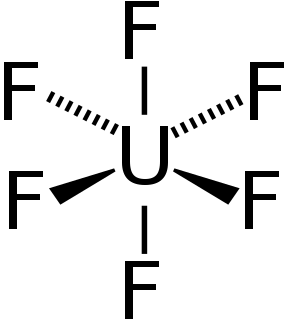
Uranium hexafluoride (UF
6), colloquially known as "hex" in the nuclear industry, is the inorganic compound with the formula UF6. Hex is a volatile white solid. It reacts with water, releasing corrosive hydrofluoric acid. The compound reacts mildly with aluminium, forming a thin surface layer of AlF3 that resists any further reaction from the compound. UF6 is used in the process of enriching uranium, which produces fuel for nuclear reactors and nuclear weapons.

Yellowcake is a type of uranium concentrate powder obtained from leach solutions, in an intermediate step in the processing of uranium ores. It is a step in the processing of uranium after it has been mined but before fuel fabrication or uranium enrichment. Yellowcake concentrates are prepared by various extraction and refining methods, depending on the types of ores. Typically, yellowcakes are obtained through the milling and chemical processing of uranium ore, forming a coarse powder that has a pungent odor, is insoluble in water, and contains about 80% uranium oxide, which melts at approximately 2880 °C.

Uranyl nitrate is a water-soluble yellow uranium salt with the formula UO2(NO3)2 · n H2O. The hexa-, tri-, and dihydrates are known. The compound is mainly of interest because it is an intermediate in the preparation of nuclear fuels.

Triuranium octoxide (U3O8) is a compound of uranium. It is present as an olive green to black, odorless solid. It is one of the more popular forms of yellowcake and is shipped between mills and refineries in this form.
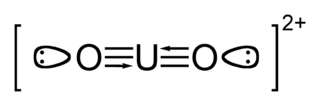
The uranyl ion is an oxycation of uranium in the oxidation state +6, with the chemical formula UO2+
2. It has a linear structure with short U–O bonds, indicative of the presence of multiple bonds between uranium and oxygen. Four or more ligands may be bound to the uranyl ion in an equatorial plane around the uranium atom. The uranyl ion forms many complexes, particularly with ligands that have oxygen donor atoms. Complexes of the uranyl ion are important in the extraction of uranium from its ores and in nuclear fuel reprocessing.

Uranium trioxide (UO3), also called uranyl oxide, uranium(VI) oxide, and uranic oxide, is the hexavalent oxide of uranium. The solid may be obtained by heating uranyl nitrate to 400 °C. Its most commonly encountered polymorph, γ-UO3, is a yellow-orange powder.
Uranyl sulfate describes a family of inorganic compounds with the formula UO2SO4(H2O)n. These salts consist of sulfate, the uranyl ion, and water. They are lemon-yellow solids. Uranyl sulfates are intermediates in some extraction methods used for uranium ores.

Ammonium diuranate or (ADU) ((NH4)2U2O7), is one of the intermediate chemical forms of uranium produced during yellowcake production. The name "yellowcake" originally given to this bright yellow salt, now applies to mixtures of uranium oxides which are actually hardly ever yellow. It also is an intermediate in mixed-oxide (MOX) fuel fabrication. Although it is usually called "ammonium diuranate" as though it has a "diuranate" ion U
2O2−
7, this is not necessarily the case. It can also be called diammonium diuranium heptaoxide. The structure is said to be similar to that of uranium dioxide dihydrate.

Sodium diuranate, Na2U2O7·6H2O, is a uranium salt also known as the yellow oxide of uranium. Sodium diuranate is commonly referred to by the initials SDU. Along with ammonium diuranate it was a component in early yellowcakes. The ratio of the two compounds is determined by process conditions; however, yellowcake is now largely a mix of uranium oxides.

Uranyl chloride refers to inorganic compounds with the formula UO2Cl2(H2O)n where n = 0, 1, or 3. These are yellow-colored solids.

A uranate is a ternary oxide involving the element uranium in one of the oxidation states 4, 5 or 6. A typical chemical formula is MxUyOz, where M represents a cation. The uranium atom in uranates(VI) has two short collinear U–O bonds and either four or six more next nearest oxygen atoms. The structures are infinite lattice structures with the uranium atoms linked by bridging oxygen atoms.

Ammonium uranyl carbonate (UO2CO3·2(NH4)2CO3) is known in the uranium processing industry as AUC and is also called uranyl ammonium carbonate. This compound is important as a component in the conversion process of uranium hexafluoride (UF6) to uranium dioxide (UO2). The ammonium uranyl carbonate is combined with steam and hydrogen at 500–600 °C to yield UO2. In another process aqueous uranyl nitrate, known as uranyl nitrate liquor (UNL) is treated with ammonium bicarbonate to form ammonium uranyl carbonate as a solid precipitate. This is separated from the solution, dried with methanol and then calcinated with hydrogen directly to UO2 to obtain a sinterable grade powder. The ex-AUC uranium dioxide powder is free-flowing, relatively coarse (10 µ) and porous with specific surface area in the range of 5 m2/g and suitable for direct pelletisation, avoiding the granulation step. Conversion to UO2 is often performed as the first stage of nuclear fuel fabrication.
Indium(III) sulfate (In2(SO4)3) is a sulfate salt of the metal indium. It is a sesquisulfate, meaning that the sulfate group occurs 11/2 times as much as the metal. It may be formed by the reaction of indium, its oxide, or its carbonate with sulfuric acid. An excess of strong acid is required, otherwise insoluble basic salts are formed. As a solid indium sulfate can be anhydrous, or take the form of a pentahydrate with five water molecules or a nonahydrate with nine molecules of water. Indium sulfate is used in the production of indium or indium containing substances. Indium sulfate also can be found in basic salts, acidic salts or double salts including indium alum.
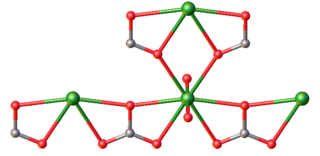
Uranyl carbonate refers to the inorganic compound with the formula UO2CO3. Also known by its mineral name rutherfordine, this material consists of uranyl (UO22+) and carbonate (CO32-). The compound is a polymeric, each uranium(VI) center being bonded to eight O atoms. Hydrolysis products of rutherfordine are also found in both the mineral and organic fractions of coal and its fly ash and is the main component of uranium in mine tailing seepage water.

Cerium is a chemical element with the symbol Ce and atomic number 58. Cerium is a soft, ductile, and silvery-white metal that tarnishes when exposed to air. Cerium is the second element in the lanthanide series, and while it often shows the +3 oxidation state characteristic of the series, it also has a stable +4 state that does not oxidize water. It is also considered one of the rare-earth elements. Cerium has no known biological role in humans but is not particularly toxic, except with intense or continued exposure.

Actinide chemistry is one of the main branches of nuclear chemistry that investigates the processes and molecular systems of the actinides. The actinides derive their name from the group 3 element actinium. The informal chemical symbol An is used in general discussions of actinide chemistry to refer to any actinide. All but one of the actinides are f-block elements, corresponding to the filling of the 5f electron shell; lawrencium, a d-block element, is also generally considered an actinide. In comparison with the lanthanides, also mostly f-block elements, the actinides show much more variable valence. The actinide series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium.
A sulfite sulfate is a chemical compound that contains both sulfite and sulfate anions [SO3]2− [SO4]2−. These compounds were discovered in the 1980s as calcium and rare earth element salts. Minerals in this class were later discovered. Minerals may have sulfite as an essential component, or have it substituted for another anion as in alloriite. The related ions [O3SOSO2]2− and [(O2SO)2SO2]2− may be produced in a reaction between sulfur dioxide and sulfate and exist in the solid form as tetramethyl ammonium salts. They have a significant partial pressure of sulfur dioxide.
References
- ↑ Hoffman, M.A.; Höschele, K. (1915). "Das Magnesiumchlorid als Mineralisator. II.: Das Urancerblau und das Wesen der konstitutiven Färbung. Das Magnesiarot und das Magnesiagrün" (PDF). Berichte. 48: 20–28. doi:10.1002/cber.19150480106.
- 1 2 Magneli, Arne; Kihlborg, Lars (1951). "On the Cerium Dioxide-Uranium Dioxide System and "Uranium Cerium Blue"" (PDF). Acta Chem. Scand. 5: 578–580. doi: 10.3891/acta.chem.scand.05-0578 .
- ↑ Passerini, L. (1930). Gazz. Chim. Ital. 60: 762.
{{cite journal}}: Missing or empty|title=(help)