
Ascomycota is a phylum of the kingdom Fungi that, together with the Basidiomycota, forms the subkingdom Dikarya. Its members are commonly known as the sac fungi or ascomycetes. It is the largest phylum of Fungi, with over 64,000 species. The defining feature of this fungal group is the "ascus", a microscopic sexual structure in which nonmotile spores, called ascospores, are formed. However, some species of the Ascomycota are asexual, meaning that they do not have a sexual cycle and thus do not form asci or ascospores. Familiar examples of sac fungi include morels, truffles, brewers' and bakers' yeast, dead man's fingers, and cup fungi. The fungal symbionts in the majority of lichens such as Cladonia belong to the Ascomycota.

Aspergillus fumigatus is a species of fungus in the genus Aspergillus, and is one of the most common Aspergillus species to cause disease in individuals with an immunodeficiency.

Chaetomium is a genus of fungi in the Chaetomiaceae family. It is a dematiaceous (dark-walled) mold normally found in soil, air, cellulose and plant debris. According to the Dictionary of the Fungi, there are about 95 species in the widespread genus.

Gibberella zeae, also known by the name of its anamorph Fusarium graminearum, is a fungal plant pathogen which causes fusarium head blight (FHB), a devastating disease on wheat and barley. The pathogen is responsible for billions of dollars in economic losses worldwide each year. Infection causes shifts in the amino acid composition of wheat, resulting in shriveled kernels and contaminating the remaining grain with mycotoxins, mainly deoxynivalenol (DON), which inhibits protein biosynthesis; and zearalenone, an estrogenic mycotoxin. These toxins cause vomiting, liver damage, and reproductive defects in livestock, and are harmful to humans through contaminated food. Despite great efforts to find resistance genes against F. graminearum, no completely resistant variety is currently available. Research on the biology of F. graminearum is directed towards gaining insight into more details about the infection process and reveal weak spots in the life cycle of this pathogen to develop fungicides that can protect wheat from scab infection.

Setosphaeria rostrata is a heat tolerant fungus with an asexual reproductive form (anamorph) known as Exserohilum rostratum. This fungus is a common plant pathogen, causing leaf spots as well as crown rot and root rot in grasses. It is also found in soils and on textiles in subtropical and tropical regions. Exserohilum rostratum is one of the 35 Exserohilum species implicated uncommonly as opportunistic pathogens of humans where it is an etiologic agent of sinusitis, keratitis, skin lesions and an often fatal meningoencephalitis. Infections caused by this species are most often seen in regions with hot climates like Israel, India and the southern USA.
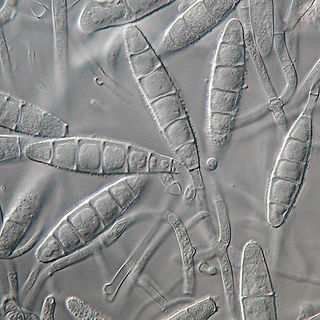
Microsporum gypseum is a soil-associated dermatophyte that occasionally is known to colonise and infect the upper dead layers of the skin of mammals. The name refers to an asexual "form-taxon" that has been associated with four related biological species of fungi: the pathogenic taxa Arthroderma incurvatum, A. gypsea, A. fulva and the non-pathogenic saprotroph A. corniculata. More recent studies have restricted M. gypseum to two teleomorphic species A. gypseum and A. incurvatum. The conidial states of A. fulva and A. corniculata have been assigned to M. fulvum and M. boullardii. Because the anamorphic states of these fungi are so similar, they can be identified reliably only by mating. Two mating strains have been discovered, "+" and "–". The classification of this species has been based on the characteristically rough-walled, blunt, club-shaped, multicelled macroconidia. Synonyms include Achorion gypseum, Microsporum flavescens, M. scorteum, and M. xanthodes. There has been past nomenclatural confusion in the usage of the generic names Microsporum and Microsporon.
Chaetomium cupreum is a fungus in the family Chaetomiaceae. It is able to decay in manufactured cellulosic materials, and is known to antagonize a wide range of soil microorganisms. This species is component of the biocontrol agent, Ketomium, a commercial biofungicide. It has also been investigated for use in the production of natural dyes. Chaetomium cupreum is mesophilic and known to occur in harsh environments and can rapidly colonize organic substrates in soil. Laboratory cultures of C. cupreum can be propagated on a range of common growth media including potato dextrose at ambient or higher than ambient temperature producing cottony white colonies with a reddish reverse.
Allomyces macrogynus is a species of fungus in the family Blastocladiaceae. It was first described by mycologist Ralph Emerson in 1941 as a variety of Allomyces javanicus, and later given distinct species status in 1954. Its genome has been sequenced by the Broad Institute.
Thielavia subthermophila is a ubiquitous, filamentous fungus that is a member of the phylum Ascomycota and order Sordariales. Known to be found on plants of arid environments, it is an endophyte with thermophilic properties, and possesses dense, pigmented mycelium. Thielavia subthermophila has rarely been identified as a human pathogen, with a small number of clinical cases including ocular and brain infections. For treatment, antifungal drugs such as amphotericin B have been used topically or intravenously, depending upon the condition.
Alternaria tenuissima is a saprophytic fungus and opportunistic plant pathogen. It is cosmopolitan in distribution, and can colonize a wide range of plant hosts. Colonies of A. tenuissima produce chains on agar growth media. The fungus often forms concentric ring patterns on infected plant leaves. This species produces the allergen Alt a 1, one of the most important outdoor seasonal fungal allergens associated with allergy and asthma provocation. In rare circumstances, this species is also known to infect immunosuppressed humans and animals.
Chaetomium atrobrunneum is a darkly pigmented mould affiliated with the fungal division, Ascomycota. This species is predominantly saprotrophic, although it has been known to infect animals including humans, showing a proclivity for the tissues of the central nervous system. Chaetomium atrobrunneum was described in 1949 from a mouldy military mattress cover obtained from the island of Guadalcanal.
Petriella setifera is a fungus commonly found in soil and feces. The fungus has also been located on wood rot, plant species, and compost. A significant portion of P. setifera reports are found on sources with no previous association with the fungus. There are no known human cases of fungal infection, but one reported case of a dolphin infection. The fungus may have immunosuppressive characteristics, but it has not been confirmed. Many properties of the fungus are unknown, requiring further research.
Botryotrichum murorum is a common soil and indoor fungus resembling members of the genus Chaetomium. The fungus has no known asexual state, and unlike many related fungi, is intolerant of high heat exhibiting limited growth when incubated at temperatures over 35 °C. In rare cases, the fungus is an opportunistic pathogen of marine animals and humans causing cutaneous and subcutaneous infection.

Penicillium spinulosum is a non-branched, fast-growing fungus with a swelling at the terminal of the stipe (vesiculate) in the genus Penicillium. P. spinulosum is able to grow and reproduce in environment with low temperature and low water availability, and is known to be acidotolerant. P. spinulosum is ubiquitously distributed, and can often be isolated from soil. Each individual strain of P. spinulosum differs from others in their colony morphology, including colony texture, amount of sporulation and roughness of conidia and conidiophores.
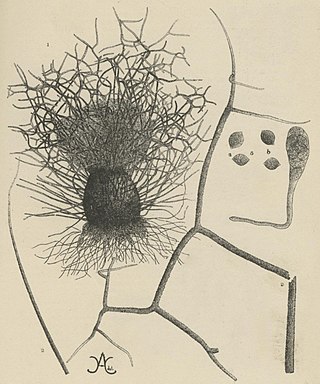
Chaetomium elatum is a very common and widely distributed saprotrophic fungus of the Chaetomiaceae family of molds which has been found to grow on many different substances all over the world. It was first established by Gustav Kunze after he observed it growing on dead leaves. Its defining features that distinguish it from other Chaetomium species are its extremely coarse terminal hairs and the lemon-shaped morphology of its ascospores. It produces many metabolites with potential biotechnology uses including one with promise against the rice blast disease fungus, Magnaporthe grisea. It shows very little pathogenic ability causing confirmed disease in only a few plant species.
Collariella bostrychodes is a fungal decomposer of lignin and carbohydrate in the family Chaetomiaceae commonly found in soil and dung. The fungus is distinguished by a darkened collar-like ostiole around the ostiolar pore, giving the fungus its name. The fungus is highly variable in shape and form, giving raise to the belief that there are two subclades in the species. The ascospores range from lemon-shaped to nearly spherical with slightly pointed ends. It can grow to be pale green and later turn pale bluish grey or olivaceous with age. The fungus produces the toxic secondary metabolite, chaetochromin.
Botryotrichum piluliferum is a fungal species first identified in 1885 by Saccardo and Marchal. It was discovered to be the asexual state of a member of the ascomycete genus, Chaetomium. The name B. piluliferum now applies to the fungus in all its states. B. piluliferum has been found worldwide in a wide range of habitats such as animal dung and vegetation. The colonies of this fungus start off white and grow rapidly to a brown colour. The conidia are smooth and white. B. piluliferum grows optimally at a temperature of 25-30 °C and a pH of 5.5.
Arcopilus aureus is a plant and soil fungus in the genus Arcopilus. It was first identified by A. H. Chivers in 1912, who named it Chaetomium aureum. It was later transferred to the genus Arcopilus by Wang and colleagues. The fungus has recently been recognized to have industrial use for the production of the metabolites resveratrol. and sclerotiorin Additionally, A. aureus has high lead tolerance and clearance, suggesting a potential role in environmental biotechnology.
Microascus manginii is a filamentous fungal species in the genus Microascus. It produces both sexual (teleomorph) and asexual (anamorph) reproductive stages known as M. manginii and Scopulariopsis candida, respectively. Several synonyms appear in the literature because of taxonomic revisions and re-isolation of the species by different researchers. M. manginii is saprotrophic and commonly inhabits soil, indoor environments and decaying plant material. It is distinguishable from closely related species by its light colored and heart-shaped ascospores used for sexual reproduction. Scopulariopsis candida has been identified as the cause of some invasive infections, often in immunocompromised hosts, but is not considered a common human pathogen. There is concern about amphotericin B resistance in S. candida.
Chaetomium perlucidum is a neurotropic dematiaceous fungus that is naturally found in the soil, including in agricultural soil, and in the stems of dead plants. The fungus can also be found on the feathers of birds, manure, seeds, and even paper. It is able to thrive at temperatures of 35 and 42 °C.








