The cerebral cortex, also known as the cerebral mantle, is the outer layer of neural tissue of the cerebrum of the brain in humans and other mammals. It is the largest site of neural integration in the central nervous system. and plays a key role in attention, perception, awareness, thought, memory, language, and consciousness. The cerebral cortex is the part of the brain responsible for cognition.

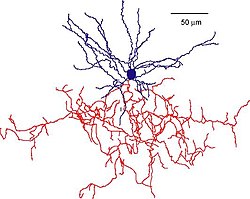
Pyramidal cells, or pyramidal neurons, are a type of multipolar neuron found in areas of the brain including the cerebral cortex, the hippocampus, and the amygdala. Pyramidal cells are the primary excitation units of the mammalian prefrontal cortex and the corticospinal tract. One of the main structural features of the pyramidal neuron is the conic shaped soma, or cell body, after which the neuron is named. Other key structural features of the pyramidal cell are a single axon, a large apical dendrite, multiple basal dendrites, and the presence of dendritic spines.
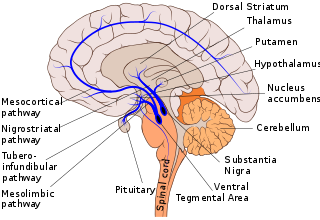
Dopaminergic pathways in the human brain are involved in both physiological and behavioral processes including movement, cognition, executive functions, reward, motivation, and neuroendocrine control. Each pathway is a set of projection neurons, consisting of individual dopaminergic neurons.

The nigrostriatal pathway is a bilateral dopaminergic pathway in the brain that connects the substantia nigra pars compacta (SNc) in the midbrain with the dorsal striatum in the forebrain. It is one of the four major dopamine pathways in the brain, and is critical in the production of movement as part of a system called the basal ganglia motor loop. Dopaminergic neurons of this pathway release dopamine from axon terminals that synapse onto GABAergic medium spiny neurons (MSNs), also known as spiny projection neurons (SPNs), located in the striatum.

The ventral tegmental area (VTA), also known as the ventral tegmental area of Tsai, or simply ventral tegmentum, is a group of neurons located close to the midline on the floor of the midbrain. The VTA is the origin of the dopaminergic cell bodies of the mesocorticolimbic dopamine system and other dopamine pathways; it is widely implicated in the drug and natural reward circuitry of the brain. The VTA plays an important role in a number of processes, including reward cognition and orgasm, among others, as well as several psychiatric disorders. Neurons in the VTA project to numerous areas of the brain, ranging from the prefrontal cortex to the caudal brainstem and several regions in between.

Interneurons are neurons that connect to brain regions, i.e. not direct motor neurons or sensory neurons. Interneurons are the central nodes of neural circuits, enabling communication between sensory or motor neurons and the central nervous system (CNS). They play vital roles in reflexes, neuronal oscillations, and neurogenesis in the adult mammalian brain.

Basket cells are inhibitory GABAergic interneurons of the brain, found throughout different regions of the cortex and cerebellum.

In neuroanatomy, thalamocortical radiations, also known as thalamocortical fibres, are the efferent fibres that project from the thalamus to distinct areas of the cerebral cortex. They form fibre bundles that emerge from the lateral surface of the thalamus.

In neuroscience, Golgi cells are the most abundant inhibitory interneurons found within the granular layer of the cerebellum. Golgi cells can be found in the granular layer at various layers. The Golgi cell is essential for controlling the activity of the granular layer. They were first identified as inhibitory in 1964. It was also the first example of an inhibitory feedback network in which the inhibitory interneuron was identified anatomically. Golgi cells produce a wide lateral inhibition that reaches beyond the afferent synaptic field and inhibit granule cells via feedforward and feedback inhibitory loops. These cells synapse onto the dendrite of granule cells and unipolar brush cells. They receive excitatory input from mossy fibres, also synapsing on granule cells, and parallel fibers, which are long granule cell axons. Thereby this circuitry allows for feed-forward and feed-back inhibition of granule cells.

The basal ganglia form a major brain system in all species of vertebrates, but in primates there are special features that justify a separate consideration. As in other vertebrates, the primate basal ganglia can be divided into striatal, pallidal, nigral, and subthalamic components. In primates, however, there are two pallidal subdivisions called the external globus pallidus (GPe) and internal globus pallidus (GPi). Also in primates, the dorsal striatum is divided by a large tract called the internal capsule into two masses named the caudate nucleus and the putamen—in most other species no such division exists, and only the striatum as a whole is recognized. Beyond this, there is a complex circuitry of connections between the striatum and cortex that is specific to primates. This complexity reflects the difference in functioning of different cortical areas in the primate brain.

In the hippocampus, the mossy fiber pathway consists of unmyelinated axons projecting from granule cells in the dentate gyrus that terminate on modulatory hilar mossy cells and in Cornu Ammonis area 3 (CA3), a region involved in encoding short-term memory. These axons were first described as mossy fibers by Santiago Ramón y Cajal as they displayed varicosities along their lengths that gave them a mossy appearance. The axons that make up the pathway emerge from the basal portions of the granule cells and pass through the hilus of the dentate gyrus before entering the stratum lucidum of CA3. Granule cell synapses tend to be glutamatergic, though immunohistological data has indicated that some synapses contain neuropeptidergic elements including opiate peptides such as dynorphin and enkephalin. There is also evidence for co-localization of both GABAergic and glutamatergic neurotransmitters within mossy fiber terminals. GABAergic and glutamatergic co-localization in mossy fiber boutons has been observed primarily in the developing hippocampus, but in adulthood, evidence suggests that mossy fiber synapses may alternate which neurotransmitter is released through activity-dependent regulation.

Parvalbumin (PV) is a calcium-binding protein with low molecular weight. In humans, it is encoded by the PVALB gene. It is a member of the albumin family; it is named for its size and its ability to coagulate.
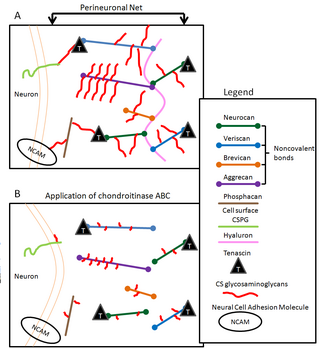
Perineuronal nets (PNNs) are specialized extracellular matrix structures responsible for synaptic stabilization in the adult brain. PNNs are found around certain neuron cell bodies and proximal neurites in the central nervous system. PNNs play a critical role in the closure of the childhood critical period, and their digestion can cause restored critical period-like synaptic plasticity in the adult brain. They are largely negatively charged and composed of chondroitin sulfate proteoglycans, molecules that play a key role in development and plasticity during postnatal development and in the adult.

GABA transporter 1 (GAT1) also known as sodium- and chloride-dependent GABA transporter 1 is a protein that in humans is encoded by the SLC6A1 gene and belongs to the solute carrier 6 (SLC6) family of transporters. It mediates gamma-aminobutyric acid's translocation from the extracellular to intracellular spaces within brain tissue and the central nervous system as a whole.

The ganglionic eminence (GE) is a transitory structure in the development of the nervous system that guides cell and axon migration. It is present in the embryonic and fetal stages of neural development found between the thalamus and caudate nucleus.

The name granule cell has been used for a number of different types of neurons whose only common feature is that they all have very small cell bodies. Granule cells are found within the granular layer of the cerebellum, the dentate gyrus of the hippocampus, the superficial layer of the dorsal cochlear nucleus, the olfactory bulb, and the cerebral cortex.
Cajal–Retzius cells are a heterogeneous population of morphologically and molecularly distinct reelin-producing cell types in the marginal zone/layer I of the developmental cerebral cortex and in the immature hippocampus of different species and at different times during embryogenesis and postnatal life.
Neurogliaform cells (NGF) are inhibitory (GABAergic) interneurons found in the cortex and the hippocampus. NGF cells represent approximately 10% of the total hippocampal inhibitory interneuron population.
An axo-axonic synapse is a type of synapse, formed by one neuron projecting its axon terminals onto another neuron's axon.
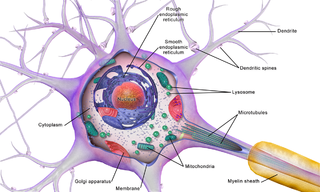
Brain cells make up the functional tissue of the brain. The rest of the brain tissue is structural or connective called the stroma which includes blood vessels. The two main types of cells in the brain are neurons, also known as nerve cells, and glial cells, also known as neuroglia.