
Titration is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte. A reagent, termed the titrant or titrator, is prepared as a standard solution of known concentration and volume. The titrant reacts with a solution of analyte to determine the analyte's concentration. The volume of titrant that reacted with the analyte is termed the titration volume.
The Winkler test is used to determine the concentration of dissolved oxygen in water samples. Dissolved oxygen (D.O.) is widely used in water quality studies and routine operation of water reclamation facilities to analyze its level of oxygen saturation.

An oxidizing agent is a substance in a redox chemical reaction that gains or "accepts"/"receives" an electron from a reducing agent. In other words, an oxidizer is any substance that oxidizes another substance. The oxidation state, which describes the degree of loss of electrons, of the oxidizer decreases while that of the reductant increases; this is expressed by saying that oxidizers "undergo reduction" and "are reduced" while reducers "undergo oxidation" and "are oxidized". Common oxidizing agents are oxygen, hydrogen peroxide, and the halogens.

Sodium hypochlorite is an alkaline inorganic chemical compound with the formula NaOCl. It is commonly known in a dilute aqueous solution as bleach or chlorine bleach. It is the sodium salt of hypochlorous acid, consisting of sodium cations and hypochlorite anions.
Chromic acid is jargon for a solution formed by the addition of sulfuric acid to aqueous solutions of dichromate. It consists at least in part of chromium trioxide.

Biochemical oxygen demand is an analytical parameter representing the amount of dissolved oxygen (DO) consumed by aerobic bacteria growing on the organic material present in a water sample at a specific temperature over a specific time period. The BOD value is most commonly expressed in milligrams of oxygen consumed per liter of sample during 5 days of incubation at 20 °C and is often used as a surrogate of the degree of organic water pollution.

Potassium permanganate is an inorganic compound with the chemical formula KMnO4. It is a purplish-black crystalline salt, which dissolves in water as K+ and MnO−
4 ions to give an intensely pink to purple solution.

Chromate salts contain the chromate anion, CrO2−
4. Dichromate salts contain the dichromate anion, Cr
2O2−
7. They are oxyanions of chromium in the +6 oxidation state and are moderately strong oxidizing agents. In an aqueous solution, chromate and dichromate ions can be interconvertible.

Potassium dichromate, K2Cr2O7, is a common inorganic chemical reagent, most commonly used as an oxidizing agent in various laboratory and industrial applications. As with all hexavalent chromium compounds, it is acutely and chronically harmful to health. It is a crystalline ionic solid with a very bright, red-orange color. The salt is popular in laboratories because it is not deliquescent, in contrast to the more industrially relevant salt sodium dichromate.
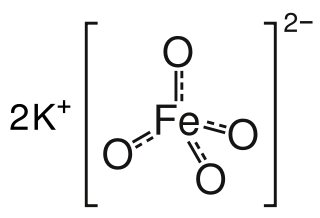
Potassium ferrate is an inorganic compound with the formula K2FeO4. It is the potassium salt of ferric acid. Potassium ferrate is a powerful oxidizing agent with applications in green chemistry, organic synthesis, and cathode technology.

Chromium trioxide is an inorganic compound with the formula CrO3. It is the acidic anhydride of chromic acid, and is sometimes marketed under the same name. This compound is a dark-purple solid under anhydrous conditions and bright orange when wet. The substance dissolves in water accompanied by hydrolysis. Millions of kilograms are produced annually, mainly for electroplating. Chromium trioxide is a powerful oxidiser, a mutagen, and a carcinogen.

Total organic carbon (TOC) is an analytical parameter representing the concentration of organic carbon in a sample. TOC determinations are made in a variety of application areas. For example, TOC may be used as a non-specific indicator of water quality, or TOC of source rock may be used as one factor in evaluating a petroleum play. For marine surface sediments average TOC content is 0.5% in the deep ocean, and 2% along the eastern margins.

Sodium dichromate is the inorganic compound with the formula Na2Cr2O7. However, the salt is usually handled as its dihydrate Na2Cr2O7·2H2O. Virtually all chromium ore is processed via conversion to sodium dichromate and virtually all compounds and materials based on chromium are prepared from this salt. In terms of reactivity and appearance, sodium dichromate and potassium dichromate are very similar. The sodium salt is, however, around twenty times more soluble in water than the potassium salt (49 g/L at 0 °C) and its equivalent weight is also lower, which is often desirable.

Wastewater quality indicators are laboratory test methodologies to assess suitability of wastewater for disposal, treatment or reuse. The main parameters in sewage that are measured to assess the sewage strength or quality as well as treatment options include: solids, indicators of organic matter, nitrogen, phosphorus, indicators of fecal contamination. Tests selected vary with the intended use or discharge location. Tests can measure physical, chemical, and biological characteristics of the wastewater. Physical characteristics include temperature and solids. Chemical characteristics include pH value, dissolved oxygen concentrations, biochemical oxygen demand (BOD) and chemical oxygen demand (COD), nitrogen, phosphorus, chlorine. Biological characteristics are determined with bioassays and aquatic toxicology tests.

Potassium hydrogen phthalate, often called simply KHP, is an acidic salt compound. It forms white powder, colorless crystals, a colorless solution, and an ionic solid that is the monopotassium salt of phthalic acid. KHP is slightly acidic, and it is often used as a primary standard for acid–base titrations because it is solid and air-stable, making it easy to weigh accurately. It is not hygroscopic. It is also used as a primary standard for calibrating pH meters because, besides the properties just mentioned, its pH in solution is very stable. It also serves as a thermal standard in thermogravimetric analysis.

Grignard reagents or Grignard compounds are chemical compounds with the general formula R−Mg−X, where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride Cl−Mg−CH3 and phenylmagnesium bromide (C6H5)−Mg−Br. They are a subclass of the organomagnesium compounds.

Chromium compounds are compounds containing the element chromium (Cr). Chromium is a member of group 6 of the transition metals. The +3 and +6 states occur most commonly within chromium compounds, followed by +2; charges of +1, +4 and +5 for chromium are rare, but do nevertheless occasionally exist.
Coffee wastewater, also known as coffee effluent, is a byproduct of coffee processing. Its treatment and disposal is an important environmental consideration for coffee processing as wastewater is a form of industrial water pollution.

The oxidation state of oxygen is −2 in almost all known compounds of oxygen. The oxidation state −1 is found in a few compounds such as peroxides. Compounds containing oxygen in other oxidation states are very uncommon: −1⁄2 (superoxides), −1⁄3 (ozonides), 0, +1⁄2 (dioxygenyl), +1, and +2.
Electro-oxidation(EO or EOx), also known as anodic oxidation or electrochemical oxidation (EC), is a technique used for wastewater treatment, mainly for industrial effluents, and is a type of advanced oxidation process (AOP). The most general layout comprises two electrodes, operating as anode and cathode, connected to a power source. When an energy input and sufficient supporting electrolyte are provided to the system, strong oxidizing species are formed, which interact with the contaminants and degrade them. The refractory compounds are thus converted into reaction intermediates and, ultimately, into water and CO2 by complete mineralization.





















