
The van Arkel–de Boer process, also known as the iodide process or crystal-bar process, was the first industrial process for the commercial production of pure ductile titanium, zirconium and some other metals. It was developed by Anton Eduard van Arkel and Jan Hendrik de Boer in 1925. Now it is used in the production of small quantities of ultrapure titanium and zirconium. It primarily involves the formation of the metal iodides and their subsequent decomposition to yield pure metal.
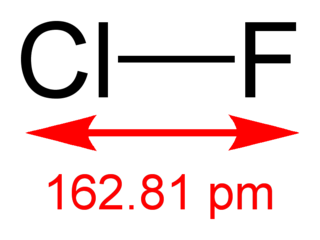
Chlorine monofluoride is a volatile interhalogen compound with the chemical formula ClF. It is a colourless gas at room temperature and is stable even at high temperatures. When cooled to −100 °C, ClF condenses as a pale yellow liquid. Many of its properties are intermediate between its parent halogens, Cl2 and F2.
Iodine pentafluoride is an interhalogen compound with chemical formula IF5. It is one of the fluorides of iodine. It is a colorless liquid, although impure samples appear yellow. It is used as a fluorination reagent and even a solvent in specialized syntheses.

Titanium tetraiodide is an inorganic compound with the formula TiI4. It is a black volatile solid, first reported by Rudolph Weber in 1863. It is an intermediate in the van Arkel–de Boer process for the purification of titanium.
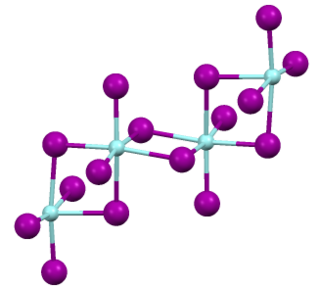
Zirconium(IV) iodide is the chemical compound with the formula ZrI4. It is the most readily available iodide of zirconium. It is an orange-coloured solid that degrades in the presence of water. The compound was once prominent as an intermediate in the purification of zirconium metal.

Titanium(II) chloride is the chemical compound with the formula TiCl2. The black solid has been studied only moderately, probably because of its high reactivity. Ti(II) is a strong reducing agent: it has a high affinity for oxygen and reacts irreversibly with water to produce H2. The usual preparation is the thermal disproportionation of TiCl3 at 500 °C. The reaction is driven by the loss of volatile TiCl4:

Gallium(III) iodide is the inorganic compound with the formula GaI3. A yellow hygroscopic solid, it is the most common iodide of gallium. In the chemical vapor transport method of growing crystals of gallium arsenide uses iodine as the transport agent. In the solid state, it exists as the dimer Ga2I6.

Tungsten dichloride dioxide, or Tungstyl chloride is the chemical compound with the formula WO2Cl2. It is a yellow-colored solid. It is used as a precursor to other tungsten compounds. Like other tungsten halides, WO2Cl2 is sensitive to moisture, undergoing hydrolysis.

Jan Hendrik de Boer was a Dutch physicist and chemist.

Potassium amide is an inorganic compound with the chemical formula KNH2. Like other alkali metal amides, it is a white solid that hydrolyzes readily. It is a strong base.

Vanadium(II) iodide is the inorganic compound with the formula VI2. It is a black micaceous solid. It adopts the cadmium iodide structure, featuring octahedral V(II) centers. The hexahydrate is also known. It forms purple crystals.

Titanium(II) iodide is the inorganic compound with the formula TiI2. It is a black micaceous solid. It adopts the cadmium iodide structure, featuring octahedral Ti(II) centers. It arises via the reaction of the elements:
The telluride iodides are chemical compounds that contain both telluride ions (Te2−) and iodide ions (I−). They are in the class of mixed anion compounds or chalcogenide halides.
Nitride fluorides containing nitride and fluoride ions with the formula NF4-. They can be electronically equivalent to a pair of oxide ions O24-. Nitride fluorides were discovered in 1996 by Lavalle et al. They heated diammonium technetium hexafluoride to 300 °C to yield TcNF. Another preparation is to heat a fluoride compound with a nitride compound in a solid state reaction. The fluorimido ion is F-N2- and is found in a rhenium compound.

In chemistry, a transition metal chloride complex is a coordination complex that consists of a transition metal coordinated to one or more chloride ligand. The class of complexes is extensive.

Vanadium (V) chloride chlorimide is a chemical compound containing vanadium in a +5 oxidation state bound to three chlorine atoms and with a double bond to a chlorimide group (=NCl). It has formula VNCl4. This can be also considered as a chloroiminato complex.
A chloride nitride is a mixed anion compound containing both chloride (Cl−) and nitride ions (N3−). Another name is metallochloronitrides. They are a subclass of halide nitrides or pnictide halides.
Arsenidogermanates are chemical compounds that contain anions with arsenic bonded to germanium. They are in the category of tetrelarsenides, pnictidogermanates, or tetrelpnictides.
Carbide chlorides are mixed anion compounds containing chloride anions and anions consisting entirely of carbon. In these compounds there is no bond between chlorine and carbon. But there is a bond between a metal and carbon. Many of these compounds are cluster compounds, in which metal atoms encase a carbon core, with chlorine atoms surrounding the cluster. The chlorine may be shared between clusters to form polymers or layers. Most carbon chloride compounds contain rare earth elements. Some are known from group 4 elements. The hexatungsten carbon cluster can be oxidised and reduced, and so have different numbers of chlorine atoms included.
Carbide iodides are mixed anion compounds containing iodide and carbide anions. Many carbide iodides are cluster compounds, containing one, two or more carbon atoms in a core, surrounded by a layer of metal atoms, encased in a shell of iodide ions. These ions may be shared between clusters to form chains, double chains or layers.














