Related Research Articles
Immunotherapy or biological therapy is the treatment of disease by activating or suppressing the immune system. Immunotherapies designed to elicit or amplify an immune response are classified as activation immunotherapies, while immunotherapies that reduce or suppress are classified as suppression immunotherapies. Immunotherapy is under preliminary research for its potential to treat various forms of cancer.
A cancer vaccine, or oncovaccine, is a vaccine that either treats existing cancer or prevents development of cancer. Vaccines that treat existing cancer are known as therapeutic cancer vaccines or tumor antigen vaccines. Some of the vaccines are "autologous", being prepared from samples taken from the patient, and are specific to that patient.

Rituximab, sold under the brand name Rituxan among others, is a monoclonal antibody medication used to treat certain autoimmune diseases and types of cancer. It is used for non-Hodgkin lymphoma, chronic lymphocytic leukemia, rheumatoid arthritis, granulomatosis with polyangiitis, idiopathic thrombocytopenic purpura, pemphigus vulgaris, myasthenia gravis and Epstein–Barr virus-positive mucocutaneous ulcers. It is given by slow intravenous infusion.

Cancer immunotherapy (immuno-oncotherapy) is the stimulation of the immune system to treat cancer, improving the immune system's natural ability to fight the disease. It is an application of the fundamental research of cancer immunology (immuno-oncology) and a growing subspecialty of oncology.

Ibritumomab tiuxetan, sold under the trade name Zevalin, is a monoclonal antibody radioimmunotherapy treatment for non-Hodgkin's lymphoma. The drug uses the monoclonal mouse IgG1 antibody ibritumomab in conjunction with the chelator tiuxetan, to which a radioactive isotope is added. Tiuxetan is a modified version of DTPA whose carbon backbone contains an isothiocyanatobenzyl and a methyl group.

Targeted therapy or molecularly targeted therapy is one of the major modalities of medical treatment (pharmacotherapy) for cancer, others being hormonal therapy and cytotoxic chemotherapy. As a form of molecular medicine, targeted therapy blocks the growth of cancer cells by interfering with specific targeted molecules needed for carcinogenesis and tumor growth, rather than by simply interfering with all rapidly dividing cells. Because most agents for targeted therapy are biopharmaceuticals, the term biologic therapy is sometimes synonymous with targeted therapy when used in the context of cancer therapy. However, the modalities can be combined; antibody-drug conjugates combine biologic and cytotoxic mechanisms into one targeted therapy.

Monoclonal antibodies (mAbs) have varied therapeutic uses. It is possible to create a mAb that binds specifically to almost any extracellular target, such as cell surface proteins and cytokines. They can be used to render their target ineffective, to induce a specific cell signal, to cause the immune system to attack specific cells, or to bring a drug to a specific cell type.

Ipilimumab, sold under the brand name Yervoy, is a monoclonal antibody medication that works to activate the immune system by targeting CTLA-4, a protein receptor that downregulates the immune system.
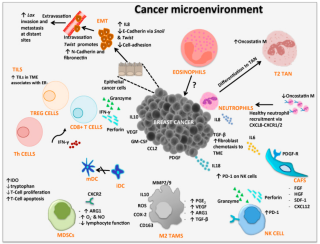
Cancer immunology (immuno-oncology) is an interdisciplinary branch of biology and a sub-discipline of immunology that is concerned with understanding the role of the immune system in the progression and development of cancer; the most well known application is cancer immunotherapy, which utilises the immune system as a treatment for cancer. Cancer immunosurveillance and immunoediting are based on protection against development of tumors in animal systems and (ii) identification of targets for immune recognition of human cancer.

A trifunctional antibody is a monoclonal antibody with binding sites for two different antigens, typically CD3 and a tumor antigen, making it a type of bispecific monoclonal antibody. In addition, its intact Fc-part can bind to an Fc receptor on accessory cells like conventional monospecific antibodies. The net effect is that this type of drug links T cells and monocytes/macrophages, natural killer cells, dendritic cells or other Fc receptor expressing cells to the tumor cells, leading to their destruction.
Gene expression profiling has revealed that diffuse large B-cell lymphoma (DLBCL) is composed of at least 3 different sub-groups, each having distinct oncogenic mechanisms that respond to therapies in different ways. Germinal Center B-Cell like (GCB) DLBCLs appear to arise from normal germinal center B cells, while Activated B-cell like (ABC) DLBCLs are thought to arise from postgerminal center B cells that are arrested during plasmacytic differentiation. The differences in gene expression between GCB DLBCL and ABC DLBCL are as vast as the differences between distinct types of leukemia, but these conditions have historically been grouped together and treated as the same disease.
Urelumab is a fully human, non‐ligand binding, CD137 agonist immunoglobulin‐γ 4 (IgG4) monoclonal antibody. It was developed utilizing Medarex's UltiMAb(R) technology by Bristol-Myers Squibb for the treatment of cancer and solid tumors. Urelumab promotes anti-tumor immunity, or an immune response against tumor cells, via CD137 activation. The application of Urelumab has been limited due to the fact that it can cause severe liver toxicity.

Nivolumab, sold under the brand name Opdivo, is an anti-cancer medication used to treat a number of types of cancer. This includes melanoma, lung cancer, malignant pleural mesothelioma, renal cell carcinoma, Hodgkin lymphoma, head and neck cancer, urothelial carcinoma, colon cancer, esophageal squamous cell carcinoma, liver cancer, gastric cancer, and esophageal or gastroesophageal junction cancer. It is administered intravenously.

The abscopal effect is a hypothesis in the treatment of metastatic cancer whereby shrinkage of untreated tumors occurs concurrently with shrinkage of tumors within the scope of the localized treatment. R.H. Mole proposed the term "abscopal" in 1953 to refer to effects of ionizing radiation "at a distance from the irradiated volume but within the same organism".
Photoimmunotherapy (PIT) is an oncological treatment that combines photodynamic therapy of tumor with immunotherapy treatment. Combining photodynamic therapy with immunotherapy enhances the immunostimulating response and has synergistic effects for metastatic cancer treatment.

Pembrolizumab, sold under the brand name Keytruda, is a humanized antibody, more specifically a PD-1 Inhibitor, used in cancer immunotherapy that treats melanoma, lung cancer, head and neck cancer, Hodgkin lymphoma, stomach cancer, cervical cancer, and certain types of breast cancer. It is administered by slow intravenous injection.

PD-1 inhibitors and PD-L1 inhibitors are a group of checkpoint inhibitor anticancer drugs that block the activity of PD-1 and PDL1 immune checkpoint proteins present on the surface of cells. Immune checkpoint inhibitors are emerging as a front-line treatment for several types of cancer.
This is a historical timeline of the development and progress of cancer treatments, which includes time of discovery, progress, and approval of the treatments.
Checkpoint inhibitor therapy is a form of cancer immunotherapy. The therapy targets immune checkpoints, key regulators of the immune system that when stimulated can dampen the immune response to an immunologic stimulus. Some cancers can protect themselves from attack by stimulating immune checkpoint targets. Checkpoint therapy can block inhibitory checkpoints, restoring immune system function. The first anti-cancer drug targeting an immune checkpoint was ipilimumab, a CTLA4 blocker approved in the United States in 2011.
Passive antibody therapy, also called serum therapy, is a subtype of passive immunotherapy that administers antibodies to target and kill pathogens or cancer cells. It is designed to draw support from foreign antibodies that are donated from a person, extracted from animals, or made in the laboratory to elicit an immune response instead of relying on the innate immune system to fight disease. It has a long history from the 18th century for treating infectious diseases and is now a common cancer treatment. The mechanism of actions include: antagonistic and agonistic reaction, complement-dependent cytotoxicity (CDC), and antibody-dependent cellular cytotoxicity (ADCC).
References
- ↑ "Definition of chemoimmunotherapy". NCI Dictionary of Cancer Terms. U.S. National Institutes of Health. One or more of the preceding sentences incorporates text from these sources, which are in the public domain :National Cancer Institute
- ↑ Emens LA (2010). "Chemoimmunotherapy". Cancer Journal. 16 (4): 295–303. doi:10.1097/PPO.0b013e3181eb5066. PMC 2919833 . PMID 20693839.
- 1 2 3 Chen G, Emens LA (February 2013). "Chemoimmunotherapy: reengineering tumor immunity". Cancer Immunology, Immunotherapy. 62 (2): 203–16. doi:10.1007/s00262-012-1388-0. PMC 3608094 . PMID 23389507.
- ↑ DeVita VT, Chu E (November 2008). "A history of cancer chemotherapy". Cancer Research. 68 (21): 8643–53. doi:10.1158/0008-5472.CAN-07-6611. PMID 18974103.
- ↑ Couzin-Frankel J (December 2013). "Breakthrough of the year 2013. Cancer immunotherapy". Science. 342 (6165). New York, N.Y.: 1432–3. doi:10.1126/science.342.6165.1432. PMID 24357284.
- ↑ Sordo-Bahamonde C, Lorenzo-Herrero S, Gonzalez-Rodriguez AP, Martínez-Pérez A, Rodrigo JP, García-Pedrero JM, et al. (May 2023). "Chemo-Immunotherapy: A New Trend in Cancer Treatment". Cancers. 15 (11): 2912. doi: 10.3390/cancers15112912 . PMC 10252089 . PMID 37296876.
- ↑ Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, et al. (January 2002). "CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma". The New England Journal of Medicine. 346 (4): 235–42. doi:10.1056/NEJMoa011795. PMID 11807147.
- ↑ Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. (March 2001). "Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2". The New England Journal of Medicine. 344 (11): 783–92. doi:10.1056/NEJM200103153441101. PMID 11248153.