A dehydrogenase is an enzyme belonging to the group of oxidoreductases that oxidizes a substrate by reducing an electron acceptor, usually NAD+/NADP+ or a flavin coenzyme such as FAD or FMN. Like all catalysts, they catalyze reverse as well as forward reactions, and in some cases this has physiological significance: for example, alcohol dehydrogenase catalyzes the oxidation of ethanol to acetaldehyde in animals, but in yeast it catalyzes the production of ethanol from acetaldehyde.

An oxidizing agent is a substance in a redox chemical reaction that gains or "accepts"/"receives" an electron from a reducing agent. In other words, an oxidizer is any substance that oxidizes another substance. The oxidation state, which describes the degree of loss of electrons, of the oxidizer decreases while that of the reductant increases; this is expressed by saying that oxidizers "undergo reduction" and "are reduced" while reducers "undergo oxidation" and "are oxidized". Common oxidizing agents are oxygen, hydrogen peroxide, and the halogens.
In biochemistry, an oxidoreductase is an enzyme that catalyzes the transfer of electrons from one molecule, the reductant, also called the electron donor, to another, the oxidant, also called the electron acceptor. This group of enzymes usually utilizes NADP+ or NAD+ as cofactors. Transmembrane oxidoreductases create electron transport chains in bacteria, chloroplasts and mitochondria, including respiratory complexes I, II and III. Some others can associate with biological membranes as peripheral membrane proteins or be anchored to the membranes through a single transmembrane helix.

Chlorate is the common name of the ClO−
3 anion, whose chlorine atom is in the +5 oxidation state. The term can also refer to chemical compounds containing this anion, with chlorates being the salts of chloric acid. Other oxyanions of chlorine can be named "chlorate" followed by a Roman numeral in parentheses denoting the oxidation state of chlorine: e.g., the ClO−
4 ion commonly called perchlorate can also be called chlorate(VII).
In enzymology, a 4-hydroxybenzoyl-CoA reductase (EC 1.3.7.9) is an enzyme found in some bacteria and archaea that catalyzes the chemical reaction
In enzymology, a 3-hydroxybenzoate 2-monooxygenase (EC 1.14.99.23) is an enzyme that catalyzes the chemical reaction
In enzymology, a deoxyhypusine monooxygenase (EC 1.14.99.29) is an enzyme that catalyzes the chemical reaction
Ecdysone 20-monooxygenase (EC 1.14.99.22) is an enzyme that catalyzes the chemical reaction
In enzymology, a linalool 8-monooxygenase (EC 1.14.14.84, Formerly EC 1.14.13.151) is an enzyme that catalyzes the chemical reaction
In enzymology, a ferredoxin-NADP+ reductase (EC 1.18.1.2) abbreviated FNR, is an enzyme that catalyzes the chemical reaction

[Methionine synthase] reductase, or Methionine synthase reductase, encoded by the gene MTRR, is an enzyme that is responsible for the reduction of methionine synthase inside human body. This enzyme is crucial for maintaining the one carbon metabolism, specifically the folate cycle. The enzyme employs one coenzyme, flavoprotein.
In enzymology, a rubredoxin-NAD+ reductase (EC 1.18.1.1) is an enzyme that catalyzes the chemical reaction.
Adenylyl-sulfate reductase (glutathione) is an enzyme that catalyzes the chemical reaction
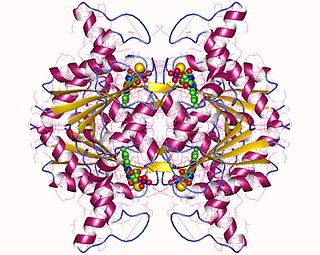
In enzymology, a NAD(P)H dehydrogenase (quinone) (EC 1.6.5.2) is an enzyme that catalyzes the chemical reaction

In enzymology, a NADPH—hemoprotein reductase is an enzyme that catalyzes the chemical reaction
Nitric oxide reductase, an enzyme, catalyzes the reduction of nitric oxide (NO) to nitrous oxide (N2O). The enzyme participates in nitrogen metabolism and in the microbial defense against nitric oxide toxicity. The catalyzed reaction may be dependent on different participating small molecules: Cytochrome c (EC: 1.7.2.5, Nitric oxide reductase (cytochrome c)), NADPH (EC:1.7.1.14), or Menaquinone (EC:1.7.5.2).
Dechloromonas agitata strain CKB is a dissimilatory perchlorate reducing bacterium (DRPB) that was isolated from paper mill waste. Strain CKB is a Gram negative, facultative anaerobe belonging to the Betaproteobacteria. The cells of strain CKB are highly motile and possess a single polar flagellum. D. agitata can couple the oxidation of several electron donors such as acetate, propionate, butyrate, lactate, succinate, fumarate, malate or yeast extract to electron acceptors such as oxygen, chlorate, perchlorate, ferrous iron, sulfide, and reduced humic substances like 2,6-anthrahydroquinone disulfonate. Unlike other perchlorate reducers, strain CKB cannot grow by nitrate reduction, which suggests that the pathways of nitrate and perchlorate reduction are distinct and unrelated, contrary to what previous research had shown.

Short-chain acyl-CoA dehydrogenase is an enzyme with systematic name short-chain acyl-CoA:electron-transfer flavoprotein 2,3-oxidoreductase. This enzyme catalyses the following chemical reaction

NADH:ubiquinone reductase (non-electrogenic) (EC 1.6.5.9, NDH-2, ubiquinone reductase, coenzyme Q reductase, dihydronicotinamide adenine dinucleotide-coenzyme Q reductase, DPNH-coenzyme Q reductase, DPNH-ubiquinone reductase, NADH-coenzyme Q oxidoreductase, NADH-coenzyme Q reductase, NADH-CoQ oxidoreductase, NADH-CoQ reductase) is an enzyme with systematic name NADH:ubiquinone oxidoreductase. This enzyme catalyses the following chemical reaction:
Perchlorate reductase is an enzyme that catalyzes the chemical reactions:







