Related Research Articles

In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons from which one hydrogen atom has been removed are functional groups called hydrocarbyls. Hydrocarbons are generally colourless and hydrophobic with only weak odours. Because of their diverse molecular structures, it is difficult to generalize further. Most anthropogenic emissions of hydrocarbons are from the burning of fossil fuels including fuel production and combustion. Natural sources of hydrocarbons such as ethylene, isoprene, and monoterpenes come from the emissions of vegetation.

A polychlorinated biphenyl (PCB) is an organic chlorine compound with the formula C12H10−xClx. Polychlorinated biphenyls were once widely deployed as dielectric and coolant fluids in electrical apparatus, carbonless copy paper and in heat transfer fluids.
Polychlorinated dibenzodioxins (PCDDs), or simply dioxins, are a group of polyhalogenated organic compounds that are significant environmental pollutants.
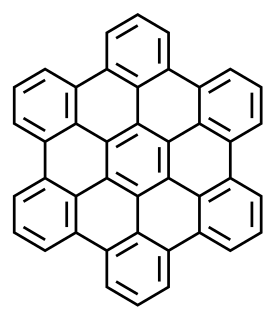
A polycyclic aromatic hydrocarbon (PAH) is a hydrocarbon—a chemical compound containing only carbon and hydrogen—that is composed of multiple aromatic rings. The group is a major subset of the aromatic hydrocarbons. The simplest of such chemicals are naphthalene, having two aromatic rings, and the three-ring compounds anthracene and phenanthrene. The terms polyaromatic hydrocarbon or polynuclear aromatic hydrocarbon are also used for this concept.
Reductive dechlorination is Chemical reaction of chlorinated organic compounds with reductants. The reaction breaks C-Cl bonds, and releases chloride ions. Many modalities have been implemented, depending on the application. Reductive dechlorination is often applied to remediation of chlorinated pesticides or dry cleaning solvents. It is also used occasionally in the synthesis of organic compounds, e.g. as pharmaceuticals.

Benzo[a]pyrene is a polycyclic aromatic hydrocarbon and the result of incomplete combustion of organic matter at temperatures between 300 °C (572 °F) and 600 °C (1,112 °F). The ubiquitous compound can be found in coal tar, tobacco smoke and many foods, especially grilled meats. The substance with the formula C20H12 is one of the benzopyrenes, formed by a benzene ring fused to pyrene. Its diol epoxide metabolites (more commonly known as BPDE) react and bind to DNA, resulting in mutations and eventually cancer. It is listed as a Group 1 carcinogen by the IARC. In the 18th century a scrotal cancer of chimney sweepers, the chimney sweeps' carcinoma, was already known to be connected to soot.
An organochloride, organochlorine compound, chlorocarbon, or chlorinated hydrocarbon is an organic compound containing at least one covalently bonded atom of chlorine that has an effect on the chemical behavior of the molecule. The chloroalkane class provides common examples. The wide structural variety and divergent chemical properties of organochlorides lead to a broad range of names and applications. Organochlorides are very useful compounds in many applications, but some are of profound environmental concern.
Persistent organic pollutants (POPs), sometimes known as "forever chemicals" are organic compounds that are resistant to environmental degradation through chemical, biological, and photolytic processes. Because of their persistence, POPs bioaccumulate with potential adverse impacts on human health and the environment. The effect of POPs on human and environmental health was discussed, with intention to eliminate or severely restrict their production, by the international community at the Stockholm Convention on Persistent Organic Pollutants in 2001.

The aryl hydrocarbon receptor is a protein that in humans is encoded by the AHR gene. The aryl hydrocarbon receptor is a transcription factor that regulates gene expression. It was originally thought to function primarily as a sensor of xenobiotic chemicals and also as the regulator of enzymes such as cytochrome P450s that metabolize these chemicals. The most notable of these xenobiotic chemicals are aromatic (aryl) hydrocarbons from which the receptor derives its name.
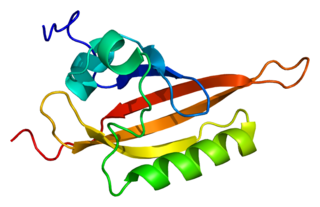
The ARNT gene encodes the aryl hydrocarbon receptor nuclear translocator protein that forms a complex with ligand-bound aryl hydrocarbon receptor (AhR), and is required for receptor function. The encoded protein has also been identified as the beta subunit of a heterodimeric transcription factor, hypoxia-inducible factor 1 (HIF1). A t(1;12)(q21;p13) translocation, which results in a TEL-ARNT fusion protein, is associated with acute myeloblastic leukemia. Three alternatively spliced variants encoding different isoforms have been described for this gene.

More thorough treatise of all groups with similar actions and binding to aryl hydrocarbon reeceptor is given in Dioxins and dioxin-like compounds.

Dioxins and dioxin-like compounds (DLCs) are a group of chemical compounds that are persistent environmental pollutants (POPs). Some of them are highly toxic, but the toxicity among them varies 30,000-fold. They are grouped together, because their mechanism of action is the same. They activate aryl hydrocarbon receptor, albeit with very different binding affinities, leading to high differences in toxicity and other effects. They are mostly by-products of burning or various industrial processes - or, in case of dioxin-like PCBs and PBBs, unwanted minor components of intentionally produced mixtures. They include:
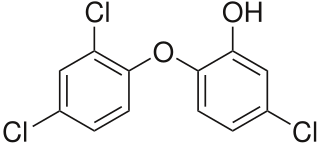
Polychloro phenoxy phenols are a group of organic polyhalogenated compounds. Among them include triclosan and predioxin which can degrade to produce certains types of dioxins and furans. Notably, however, the particular dioxin formed by degradation of triclosan, 2,8-DCDD, was found to be non-toxic in fish embryos.

The environmental impact of paper is significant, which has led to changes in industry and behaviour at both business and personal levels. With the use of modern technology such as the printing press and the highly mechanized harvesting of wood, disposable paper became a relatively cheap commodity, which led to a high level of consumption and waste. The rise in global environmental issues such as air and water pollution, climate change, overflowing landfills and clearcutting have all lead to increased government regulations. There is now a trend towards sustainability in the pulp and paper industry as it moves to reduce clear cutting, water use, greenhouse gas emissions, fossil fuel consumption and clean up its impacts on local water supplies and air pollution.

2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) is a polychlorinated dibenzo-p-dioxin with the chemical formula C
12H
4Cl
4O
2. Pure TCDD is a colorless solid with no distinguishable odor at room temperature. It is usually formed as an unwanted product in burning processes of organic materials or as a side product in organic synthesis.

Pentachlorobenzene (PeCB) is a chemical compound with the molecular formula C6HCl5 which is a chlorinated aromatic hydrocarbon. It consists of a benzene ring substituted with five chlorine atoms. PeCB was once used industrially for a variety of uses, but because of environmental concerns there are currently no large scale uses of PeCB. Pentachlorobenzene is a known persistent organic pollutant (POP) and banned globally by the Stockholm Convention on Persistent Organic Pollutants in 2009.
Toxic equivalency factor (TEF) expresses the toxicity of dioxins, furans and PCBs in terms of the most toxic form of dioxin, 2,3,7,8-TCDD. The toxicity of the individual congeners may vary by orders of magnitude.

Dibenzopyrenes are a group of high molecular weight polycyclic aromatic hydrocarbons with the molecular formula C24H14. There are five isomers of dibenzopyrene which differ by the arrangement of aromatic rings: dibenzo[a,e]pyrene, dibenzo[a,h]pyrene, dibenzo[a,i]pyrene, dibenzo[a,l]pyrene, and dibenzo[e,l]pyrene.
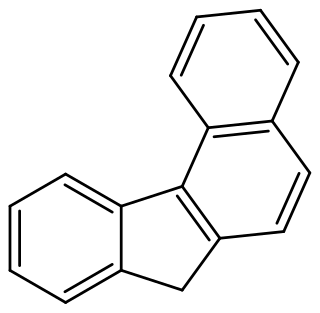
Benzo[c]fluorene is a polycyclic aromatic hydrocarbon (PAH) with mutagenic activity. It is a component of coal tar, cigarette smoke and smog and thought to be a major contributor to its carcinogenic properties. The mutagenicity of benzo[c]fluorene is mainly attributed to formation of metabolites that are reactive and capable of forming DNA adducts. According to the KEGG it is a group 3 carcinogen. Other names for benzo[c]fluorene are 7H-benzo[c]fluorene, 3,4-benzofluorene, and NSC 89264.

1,2,3,4,6,7,8-Heptachlorodibenzo-para-dioxin (often referred to as 1,2,3,4,6,7,8-HpCDD) is a polychlorinated derivative of dibenzo-p-dioxin and can therefore be categorized as polychlorinated dibenzo-p-dioxin (PCDD), a subclass of dioxins which includes 75 congeners. HpCDD is the dibenzo-p-dioxin which is chlorinated at positions 1, 2, 3, 4, 6, 7, and 8. It is a polycyclic heterocyclic organic compound, since HpCDD contains multiple cyclic structures (two benzene rings connected by a 1,4-dioxin ring) in which two different elements (carbon and oxygen) are members of its rings. HpCDD has molecular formula C12HCl7O2 and is an off-white powder, which is insoluble in water.
References
- 1 2 3 Nilsson, U. L.; Oestman, C. E. (1993). "Chlorinated polycyclic aromatic hydrocarbons: Method of analysis and their occurrence in urban air". Environmental Science & Technology. 27 (9): 1826. Bibcode:1993EnST...27.1826N. doi:10.1021/es00046a010.
- 1 2 Kitazawa, A.; Amagai, T.; Ohura, T. (2006). "Temporal Trends and Relationships of Particulate Chlorinated Polycyclic Aromatic Hydrocarbons and Their Parent Compounds in Urban Air". Environmental Science & Technology. 40 (15): 4592–8. Bibcode:2006EnST...40.4592K. doi:10.1021/es0602703. PMID 16913111.
- ↑ Cerniglia, C. E. (1992). "Biodegradation of polycyclic aromatic hydrocarbons". Biodegradation. 3 (2–3): 351–368. doi:10.1007/BF00129093. S2CID 25516145.
- 1 2 3 Ohura, T.; Fujima, S.; Amagai, T.; Shinomiya, M. (2008). "Chlorinated Polycyclic Aromatic Hydrocarbons in the Atmosphere: Seasonal Levels, Gas-Particle Partitioning, and Origin". Environmental Science & Technology. 42 (9): 3296–302. Bibcode:2008EnST...42.3296O. doi:10.1021/es703068n. PMID 18522109.
- 1 2 Ohura, T. (2007). "Environmental Behavior, Sources, and Effects of Chlorinated Polycyclic Aromatic Hydrocarbons". The Scientific World Journal. 7: 372–380. doi:10.1100/tsw.2007.75. PMC 5900950 . PMID 17334629.
- ↑ Ma, J.; Horii, Y.; Cheng, J.; Wang, W.; Wu, Q.; Ohura, T.; Kannan, K. (2009). "Chlorinated and Parent Polycyclic Aromatic Hydrocarbons in Environmental Samples from an Electronic Waste Recycling Facility and a Chemical Industrial Complex in China". Environmental Science & Technology. 43 (3): 643–9. Bibcode:2009EnST...43..643M. doi:10.1021/es802878w. PMID 19244996.
- ↑ Wang, D.; Xu, X.; Chu, S.; Zhang, D. (2003). "Analysis and structure prediction of chlorinated polycyclic aromatic hydrocarbons released from combustion of polyvinylchloride". Chemosphere. 53 (5): 495–503. Bibcode:2003Chmsp..53..495W. doi:10.1016/S0045-6535(03)00507-1. PMID 12948533.
- ↑ Horii, Y.; Ok, G.; Ohura, T.; Kannan, K. (2008). "Occurrence and Profiles of Chlorinated and Brominated Polycyclic Aromatic Hydrocarbons in Waste Incinerators". Environmental Science & Technology. 42 (6): 1904–9. Bibcode:2008EnST...42.1904H. doi:10.1021/es703001f. PMID 18409611.
- ↑ Horii, Y.; Khim, J. S.; Higley, E. B.; Giesy, J. P.; Ohura, T.; Kannan, K. (2009). "Relative Potencies of Individual Chlorinated and Brominated Polycyclic Aromatic Hydrocarbons for Induction of Aryl Hydrocarbon Receptor-Mediated Responses". Environmental Science & Technology. 43 (6): 2159. Bibcode:2009EnST...43.2159H. doi:10.1021/es8030402. PMID 19368229.
- ↑ Huang, Chao; Xu, Xiong; Wang, Donghong; Ma, Mei; Rao, Kaifeng; Wang, Zijian (2018). "The aryl hydrocarbon receptor (AhR) activity and DNA-damaging effects of chlorinated polycyclic aromatic hydrocarbons (Cl-PAHs)". Chemosphere. 211: 640–647. Bibcode:2018Chmsp.211..640H. doi:10.1016/j.chemosphere.2018.07.087. PMID 30098559.
- ↑ Blankenship, A. L.; Kannan, K.; Villalobos, S. A.; Villeneuve, D. L.; Falandysz, J.; Imagawa, T.; Jakobsson, E.; Giesy, J. P. (2000). "Relative Potencies of Individual Polychlorinated Naphthalenes and Halowax Mixtures to Induce Ah Receptor-Mediated Responses". Environmental Science & Technology. 34 (15): 3153. Bibcode:2000EnST...34.3153B. doi:10.1021/es9914339.
- ↑ Ohura, T.; Kitazawa, A.; Amagai, T.; Makino, M. (2005). "Occurrence, Profiles, and Photostabilities of Chlorinated Polycyclic Aromatic Hydrocarbons Associated with Particulates in Urban Air". Environmental Science & Technology. 39 (1): 85–91. Bibcode:2005EnST...39...85O. doi:10.1021/es040433s. PMID 15667079.