Silane is an inorganic compound with chemical formula, SiH4. It is a colourless, pyrophoric, toxic gas with a sharp, repulsive smell, somewhat similar to that of acetic acid. Silane is of practical interest as a precursor to elemental silicon. Silane with alkyl groups are effective water repellents for mineral surfaces such as concrete and masonry. Silanes with both organic and inorganic attachments are used as coupling agents.

Trichlorosilane is an inorganic compound with the formula HCl3Si. It is a colourless, volatile liquid. Purified trichlorosilane is the principal precursor to ultrapure silicon in the semiconductor industry. In water, it rapidly decomposes to produce a siloxane polymer while giving off hydrochloric acid. Because of its reactivity and wide availability, it is frequently used in the synthesis of silicon-containing organic compounds.
In chemistry, an interhalogen compound is a molecule which contains two or more different halogen atoms and no atoms of elements from any other group.
Chlorine trifluoride is an interhalogen compound with the formula ClF3. This colorless, poisonous, corrosive, and extremely reactive gas condenses to a pale-greenish yellow liquid, the form in which it is most often sold (pressurized at room temperature). The compound is primarily of interest in plasmaless cleaning and etching operations in the semiconductor industry, in nuclear reactor fuel processing, historically as a component in rocket fuels, and various other industrial operations owing to its corrosive nature.

Disulfur decafluoride is a chemical compound with the formula S2F10. It was discovered in 1934 by Denbigh and Whytlaw-Gray. Each sulfur atom of the S2F10 molecule is octahedral, and surrounded by five fluorine atoms and one sulfur atom. The two sulfur atoms are connected by a single bond. In the S2F10 molecule, the oxidation state of each sulfur atoms is +5, but their valency is 6. S2F10 is highly toxic, with toxicity four times that of phosgene.

Xenon difluoride is a powerful fluorinating agent with the chemical formula XeF
2, and one of the most stable xenon compounds. Like most covalent inorganic fluorides it is moisture-sensitive. It decomposes on contact with water vapor, but is otherwise stable in storage. Xenon difluoride is a dense, colourless crystalline solid.

Sulfur tetrafluoride is the chemical compound with the formula SF4. It is a colorless corrosive gas that releases dangerous HF upon exposure to water or moisture. Despite these unwelcome characteristics, this compound is a useful reagent for the preparation of organofluorine compounds, some of which are important in the pharmaceutical and specialty chemical industries.

Selenium tetrafluoride (SeF4) is an inorganic compound. It is a colourless liquid that reacts readily with water. It can be used as a fluorinating reagent in organic syntheses (fluorination of alcohols, carboxylic acids or carbonyl compounds) and has advantages over sulfur tetrafluoride in that milder conditions can be employed and it is a liquid rather than a gas.

Silicon monoxide is the chemical compound with the formula SiO where silicon is present in the oxidation state +2. In the vapour phase, it is a diatomic molecule. It has been detected in stellar objects and has been described as the most common oxide of silicon in the universe.
Boron monofluoride or fluoroborylene is a chemical compound with formula BF, one atom of boron and one of fluorine. It was discovered as an unstable gas and only in 2009 found to be a stable ligand combining with transition metals, in the same way as carbon monoxide. It is a subhalide, containing fewer than the normal number of fluorine atoms, compared with boron trifluoride. It can also be called a borylene, as it contains boron with two unshared electrons. BF is isoelectronic with carbon monoxide and dinitrogen; each molecule has 14 electrons.

Thiophosphoryl fluoride is an inorganic molecular gas with formula PSF3 containing phosphorus, sulfur and fluorine. It spontaneously ignites in air and burns with a cool flame. The discoverers were able to have flames around their hands without discomfort, and called it "probably one of the coldest flames known". The gas was discovered in 1888.
Fluorine forms a great variety of chemical compounds, within which it always adopts an oxidation state of −1. With other atoms, fluorine forms either polar covalent bonds or ionic bonds. Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two examples of a higher order bond exist. Fluoride may act as a bridging ligand between two metals in some complex molecules. Molecules containing fluorine may also exhibit hydrogen bonding. Fluorine's chemistry includes inorganic compounds formed with hydrogen, metals, nonmetals, and even noble gases; as well as a diverse set of organic compounds. For many elements the highest known oxidation state can be achieved in a fluoride. For some elements this is achieved exclusively in a fluoride, for others exclusively in an oxide; and for still others the highest oxidation states of oxides and fluorides are always equal.
Boron monofluoride monoxide or oxoboryl fluoride or fluoroxoborane is an unstable inorganic molecular substance with formula FBO. It is also called boron fluoride oxide, fluoro(oxo)borane or fluoro-oxoborane. The molecule is stable at high temperatures, but below 1000 °C condenses to a trimer (BOF)3 called trifluoroboroxin. FBO can be isolated as a triatomic non-metallic molecule in an inert gas matrix, and has been condensed in solid neon and argon. When an attempt is made to condense the gas to a solid in bulk, a polymeric glass is formed, which is deficient in fluoride, and when heated forms a glassy froth like popcorn. Boron fluoride oxide has been studied because of its production in high energy rocket fuels that contain boron and fluorine, and in the form of an oxyfluoride glass. BOF glass is unusual in that it can condense directly from gas.
Difluoroamino sulfur pentafluoride is a gaseous chemical compound of fluorine, sulfur, and nitrogen. It is unusual in having a hexa-coordinated sulfur atom with a link to nitrogen. Other names for this substance include difluoro(pentafluorosulfur)amine, pentafluorosulfanyldifluoramine, and pentafluorosulfanyl N,N-difluoramine.
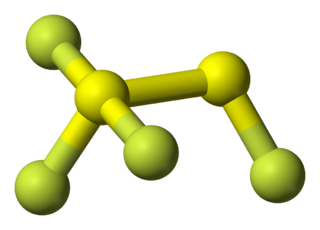
1,1,1,2-tetrafluorodisulfane, also known as 1,2-difluorodisulfane 1,1-difluoride or just difluorodisulfanedifluoride (FSSF3) is an unstable molecular compound of fluorine and sulfur. The molecule has a pair of sulfur atoms, with one fluorine atom on one sulfur, and three fluorine atoms on the other. It has the uncommon property that all the bond lengths are different. The bond strength is not correlated with bond length but is inversely correlated with the force constant (Badger's rule). The molecule can be considered as sulfur tetrafluoride in which a sulfur atom is inserted into a S-F bond.
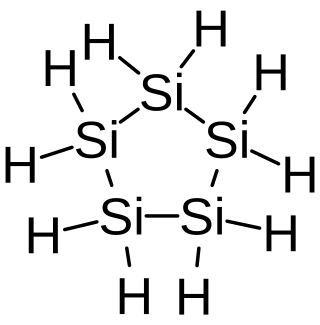
Cyclopentasilane is a cyclic compound of silicon and hydrogen. Containing five silicon atoms arranged in a ring, it is the silicon analog of cyclopentane. Cyclopentasilane is a liquid oligosilane. It is of research interest because of its potential use as a liquid silicon ink for printing silicon structures on integrated circuits or solar cells.
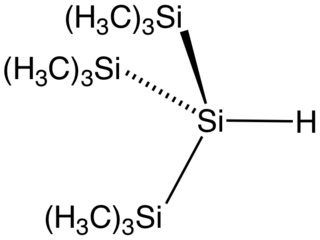
Tris(trimethylsilyl)silane is the organosilicon compound with the formula (Me3Si)3SiH (where Me = CH3). It is a colorless liquid that is classified as a hydrosilane since it contains an Si-H bond. The compound is notable as having a weak Si-H bond, with a bond dissociation energy estimated at 84 kcal/mol. For comparison, the Si-H bond in trimethylsilane is 94 kcal/mol. With such a weak bond, the compound is used as a reagent to deliver hydrogen atoms. The compound has been described as an environmentally benign analogue of tributyltin hydride.

Difluorosilane is a gaseous chemical compound with formula SiH2F2. It can be considered as a derivative of silane with two hydrogen atoms replaced with fluorine.

Chlorine oxide trifluoride or chlorine trifluoride oxide is a corrosive liquid molecular compound with formula ClOF3. It was developed secretly as a rocket fuel oxidiser.
Diphosphorus tetrafluoride is a gaseous compound of phosphorus and fluorine with formula P2F4. Two fluorine atoms are connected to each phosphorus atom, and there is a bond between the two phosphorus atoms. Phosphorus can be considered to have oxidation state +2, as indicated by the name phosphorus difluoride.











