Mesitylene or 1,3,5-trimethylbenzene is a derivative of benzene with three methyl substituents positioned symmetrically around the ring. The other two isomeric trimethylbenzenes are 1,2,4-trimethylbenzene (pseudocumene) and 1,2,3-trimethylbenzene (hemimellitene). All three compounds have the formula C6H3(CH3)3, which is commonly abbreviated C6H3Me3. Mesitylene is a colorless liquid with sweet aromatic odor. It is a component of coal tar, which is its traditional source. It is a precursor to diverse fine chemicals. The mesityl group (Mes) is a substituent with the formula C6H2Me3 and is found in various other compounds.

Acrylic acid (IUPAC: propenoic acid) is an organic compound with the formula CH2=CHCOOH. It is the simplest unsaturated carboxylic acid, consisting of a vinyl group connected directly to a carboxylic acid terminus. This colorless liquid has a characteristic acrid or tart smell. It is miscible with water, alcohols, ethers, and chloroform. More than a million tons are produced annually.
In organic chemistry, hydroformylation, also known as oxo synthesis or oxo process, is an industrial process for the production of aldehydes from alkenes. This chemical reaction entails the net addition of a formyl group and a hydrogen atom to a carbon-carbon double bond. This process has undergone continuous growth since its invention: production capacity reached 6.6×106 tons in 1995. It is important because aldehydes are easily converted into many secondary products. For example, the resultant aldehydes are hydrogenated to alcohols that are converted to detergents. Hydroformylation is also used in speciality chemicals, relevant to the organic synthesis of fragrances and pharmaceuticals. The development of hydroformylation is one of the premier achievements of 20th-century industrial chemistry.
In chemistry, homogeneous catalysis is catalysis where the catalyst is in same phase as reactants, principally by a soluble catalyst a in solution. In contrast, heterogeneous catalysis describes processes where the catalysts and substrate are in distinct phases, typically solid-gas, respectively. The term is used almost exclusively to describe solutions and implies catalysis by organometallic compounds. Homogeneous catalysis is an established technology that continues to evolve. An illustrative major application is the production of acetic acid. Enzymes are examples of homogeneous catalysts.

Chromium(III) chloride (also called chromic chloride) is an inorganic chemical compound with the chemical formula CrCl3. It forms several hydrates with the formula CrCl3·nH2O, among which are hydrates where n can be 5 (chromium(III) chloride pentahydrate CrCl3·5H2O) or 6 (chromium(III) chloride hexahydrate CrCl3·6H2O). The anhydrous compound with the formula CrCl3 are violet crystals, while the most common form of the chromium(III) chloride are the dark green crystals of hexahydrate, CrCl3·6H2O. Chromium chlorides find use as catalysts and as precursors to dyes for wool.
An alkyne trimerisation is a [2+2+2] cycloaddition reaction in which three alkyne units react to form a benzene ring. The reaction requires a metal catalyst. The process is of historic interest as well as being applicable to organic synthesis. Being a cycloaddition reaction, it has high atom economy. Many variations have been developed, including cyclisation of mixtures of alkynes and alkenes as well as alkynes and nitriles.

1-Hexene (hex-1-ene) is an organic compound with the formula C6H12. It is an alkene that is classified in industry as higher olefin and an alpha-olefin, the latter term meaning that the double bond is located at the alpha (primary) position, endowing the compound with higher reactivity and thus useful chemical properties. 1-Hexene is an industrially significant linear alpha olefin. 1-Hexene is a colourless liquid.
Ethylenediamine (abbreviated as en when a ligand) is the organic compound with the formula C2H4(NH2)2. This colorless liquid with an ammonia-like odor is a basic amine. It is a widely used building block in chemical synthesis, with approximately 500,000 tonnes produced in 1998. Ethylenediamine is the first member of the so-called polyethylene amines.

In organometallic chemistry, a metallacycle is a derivative of a carbocyclic compound wherein a metal has replaced at least one carbon center; this is to some extent similar to heterocycles. Metallacycles appear frequently as reactive intermediates in catalysis, e.g. olefin metathesis and alkyne trimerization. In organic synthesis, directed ortho metalation is widely used for the functionalization of arene rings via C-H activation. One main effect that metallic atom substitution on a cyclic carbon compound is distorting the geometry due to the large size of typical metals.

The Nozaki–Hiyama–Kishi reaction is a nickel/chromium coupling reaction forming an alcohol from the reaction of an aldehyde with an allyl or vinyl halide. In their original 1977 publication, Tamejiro Hiyama and Hitoshi Nozaki reported on a chromium(II) salt solution prepared by reduction of chromic chloride by lithium aluminium hydride to which was added benzaldehyde and allyl chloride:
Organochromium chemistry is a branch of organometallic chemistry that deals with organic compounds containing a chromium to carbon bond and their reactions. The field is of some relevance to organic synthesis. The relevant oxidation states for organochromium complexes encompass the entire range of possible oxidation states from –4 (d10) in Na4[Cr–IV(CO)4] to +6 (d0) in oxo-alkyl complexes like Cp*CrVI(=O)2Me.

Manganese(II,III) oxide is the chemical compound with formula Mn3O4. Manganese is present in two oxidation states +2 and +3 and the formula is sometimes written as MnO·Mn2O3. Mn3O4 is found in nature as the mineral hausmannite.

Acetic acid, systematically named ethanoic acid, is an acidic, colourless liquid and organic compound with the chemical formula CH3COOH. Vinegar is at least 4% acetic acid by volume, making acetic acid the main component of vinegar apart from water. It has been used, as a component of vinegar, throughout history from at least the third century BC.

In chemistry, a catalyst support is a material, usually a solid with a high surface area, to which a catalyst is affixed. The activity of heterogeneous catalysts is mainly promoted by atoms present at the accessible surface of the material. Consequently, great effort is made to maximize the specific surface area of a catalyst. One popular method for increasing surface area involves distributing the catalyst over the surface of the support. The support may be inert or participate in the catalytic reactions. Typical supports include various kinds of activated carbon, alumina, and silica.

A metal salen complex is a coordination compound between a metal cation and a ligand derived from N,N′-bis(salicylidene)ethylenediamine, commonly called salen. The classical example is salcomine, the complex with divalent cobalt Co2+, usually denoted as Co(salen). These complexes are widely investigated as catalysts and enzyme mimics.

Chromium(III) phosphate describes inorganic compounds with the chemical formula CrPO4·(H2O)n, where n = 0, 4, or 6. All are deeply colored solids. Anhydrous CrPO4 is green. The hexahydrate CrPO4·6H2O is violet.

The Phillips catalyst, or the Phillips supported chromium catalyst, is the catalyst used to produce approximately half of the world's polyethylene. A heterogeneous catalyst, it consists of a chromium oxide supported on silica gel. Polyethylene, the most-produced synthetic polymer, is produced industrially by the polymerization of ethylene:

Di-tert-butyl chromate is an alkoxide with the formula CrO2(OC(CH3)3)2. It is prepared by treatment of t-butanol with chromic anhydride. It forms red crystals at temperatures below –5 °C, above which it melts to give a red oil. The complex, which is diamagnetic, is of fundamental interest as a model for the intermediates in oxidations of alcohols by chromium(VI). This complex is stable because as a t-butyl groups lack beta-hydrogens. This complex and its analogues have tetrahedral geometry at chromium, as established by X-ray crystallography of its analogues.
In organic chemistry, hydrovinylation is the formal insertion of an alkene into the C-H bond of ethylene. The more general reaction, hydroalkenylation, is the formal insertion of an alkene into the C-H bond of any terminal alkene. The reaction is catalyzed by metal complexes. A representative reaction is the conversion of styrene and ethylene to 3-phenybutene:
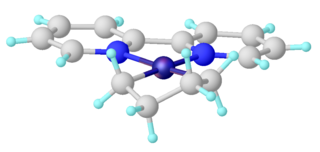
In organometallic chemistry, metallacyclopentanes are compounds with the formula LnM(CH2)4 (Ln = ligands, and M = metal). They are a type of metallacycle. Metallacyclopentanes are intermediates in some metal-catalysed reactions in homogeneous catalysis.















