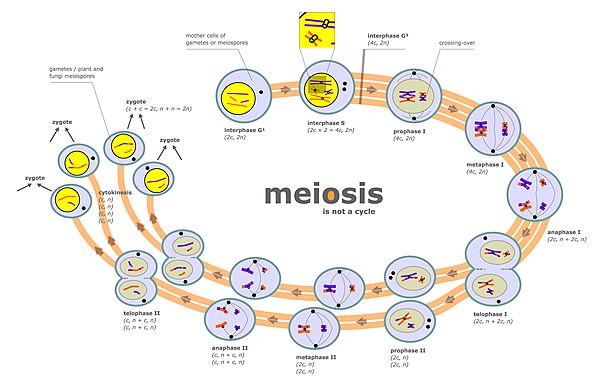
Meiosis is a special type of cell division of germ cells in sexually-reproducing organisms that produces the gametes, the sperm or egg cells. It involves two rounds of division that ultimately result in four cells, each with only one copy of each chromosome (haploid). Additionally, prior to the division, genetic material from the paternal and maternal copies of each chromosome is crossed over, creating new combinations of code on each chromosome. Later on, during fertilisation, the haploid cells produced by meiosis from a male and a female will fuse to create a zygote, a cell with two copies of each chromosome again.

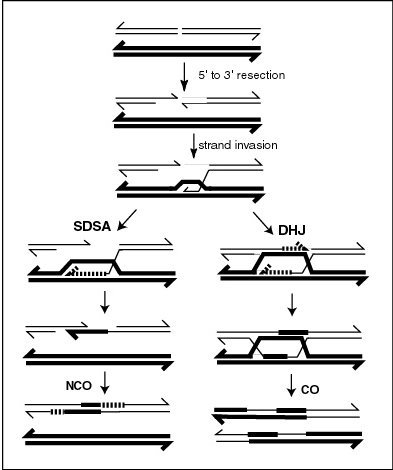
Chromosomal crossover, or crossing over, is the exchange of genetic material during sexual reproduction between two homologous chromosomes' non-sister chromatids that results in recombinant chromosomes. It is one of the final phases of genetic recombination, which occurs in the pachytene stage of prophase I of meiosis during a process called synapsis. Synapsis begins before the synaptonemal complex develops and is not completed until near the end of prophase I. Crossover usually occurs when matching regions on matching chromosomes break and then reconnect to the other chromosome.
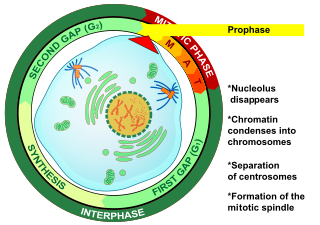
Prophase is the first stage of cell division in both mitosis and meiosis. Beginning after interphase, DNA has already been replicated when the cell enters prophase. The main occurrences in prophase are the condensation of the chromatin reticulum and the disappearance of the nucleolus.

Genetic recombination is the exchange of genetic material between different organisms which leads to production of offspring with combinations of traits that differ from those found in either parent. In eukaryotes, genetic recombination during meiosis can lead to a novel set of genetic information that can be further passed on from parents to offspring. Most recombination occurs naturally and can be classified into two types: (1) interchromosomal recombination, occurring through independent assortment of alleles whose loci are on different but homologous chromosomes ; & (2) intrachromosomal recombination, occurring through crossing over.

A pair of homologous chromosomes, or homologs, is a set of one maternal and one paternal chromosome that pair up with each other inside a cell during fertilization. Homologs have the same genes in the same loci, where they provide points along each chromosome that enable a pair of chromosomes to align correctly with each other before separating during meiosis. This is the basis for Mendelian inheritance, which characterizes inheritance patterns of genetic material from an organism to its offspring parent developmental cell at the given time and area.

Nondisjunction is the failure of homologous chromosomes or sister chromatids to separate properly during cell division (mitosis/meiosis). There are three forms of nondisjunction: failure of a pair of homologous chromosomes to separate in meiosis I, failure of sister chromatids to separate during meiosis II, and failure of sister chromatids to separate during mitosis. Nondisjunction results in daughter cells with abnormal chromosome numbers (aneuploidy).

A heteroduplex is a double-stranded (duplex) molecule of nucleic acid originated through the genetic recombination of single complementary strands derived from different sources, such as from different homologous chromosomes or even from different organisms.

The synaptonemal complex (SC) is a protein structure that forms between homologous chromosomes during meiosis and is thought to mediate synapsis and recombination during prophase I during meiosis in eukaryotes. It is currently thought that the SC functions primarily as a scaffold to allow interacting chromatids to complete their crossover activities.

Synapsis or Syzygy is the pairing of two chromosomes that occurs during meiosis. It allows matching-up of homologous pairs prior to their segregation, and possible chromosomal crossover between them. Synapsis takes place during prophase I of meiosis. When homologous chromosomes synapse, their ends are first attached to the nuclear envelope. These end-membrane complexes then migrate, assisted by the extranuclear cytoskeleton, until matching ends have been paired. Then the intervening regions of the chromosome are brought together, and may be connected by a protein-DNA complex called the synaptonemal complex. During synapsis, autosomes are held together by the synaptonemal complex along their whole length, whereas for sex chromosomes, this only takes place at one end of each chromosome.
Zygotene is the second stage of prophase I during meiosis, the specialized cell division that reduces the chromosome number by half to produce haploid gametes. It follows the Leptotene stage.
The pachytene stage, also known as pachynema, is the third stage of prophase I during meiosis, the specialized cell division that reduces chromosome number by half to produce haploid gametes. It follows the zygotene stage.
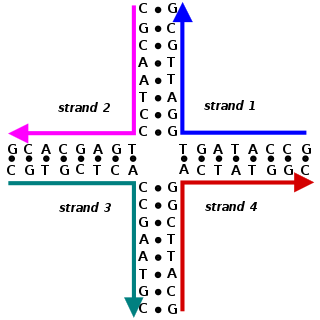
A Holliday junction is a branched nucleic acid structure that contains four double-stranded arms joined. These arms may adopt one of several conformations depending on buffer salt concentrations and the sequence of nucleobases closest to the junction. The structure is named after Robin Holliday, the molecular biologist who proposed its existence in 1964.
Mitotic recombination is a type of genetic recombination that may occur in somatic cells during their preparation for mitosis in both sexual and asexual organisms. In asexual organisms, the study of mitotic recombination is one way to understand genetic linkage because it is the only source of recombination within an individual. Additionally, mitotic recombination can result in the expression of recessive alleles in an otherwise heterozygous individual. This expression has important implications for the study of tumorigenesis and lethal recessive alleles. Mitotic homologous recombination occurs mainly between sister chromatids subsequent to replication. Inter-sister homologous recombination is ordinarily genetically silent. During mitosis the incidence of recombination between non-sister homologous chromatids is only about 1% of that between sister chromatids.

Sister chromatid exchange (SCE) is the exchange of genetic material between two identical sister chromatids.

MutS protein homolog 5 is a protein that in humans is encoded by the MSH5 gene.

MutS protein homolog 4 is a protein that in humans is encoded by the MSH4 gene.

DNA mismatch repair protein Mlh3 is a protein that in humans is encoded by the MLH3 gene.

The meiotic recombination checkpoint monitors meiotic recombination during meiosis, and blocks the entry into metaphase I if recombination is not efficiently processed.
The leptotene stage, also known as leptonema, is the first of five substages of prophase I during meiosis, the specialized cell division that reduces the chromosome number by half to produce haploid gametes in sexually reproducing organisms.
The origin and function of meiosis are currently not well understood scientifically, and would provide fundamental insight into the evolution of sexual reproduction in eukaryotes. There is no current consensus among biologists on the questions of how sex in eukaryotes arose in evolution, what basic function sexual reproduction serves, and why it is maintained, given the basic two-fold cost of sex. It is clear that it evolved over 1.2 billion years ago, and that almost all species which are descendants of the original sexually reproducing species are still sexual reproducers, including plants, fungi, and animals.