
In chemistry, particularly in biochemistry, a fatty acid is a carboxylic acid with an aliphatic chain, which is either saturated or unsaturated. Most naturally occurring fatty acids have an unbranched chain of an even number of carbon atoms, from 4 to 28. Fatty acids are a major component of the lipids in some species such as microalgae but in some other organisms are not found in their standalone form, but instead exist as three main classes of esters: triglycerides, phospholipids, and cholesteryl esters. In any of these forms, fatty acids are both important dietary sources of fuel for animals and important structural components for cells.

In nutrition, biology, and chemistry, fat usually means any ester of fatty acids, or a mixture of such compounds, most commonly those that occur in living beings or in food.
Omega−3 fatty acids, also called Omega−3 oils, ω−3 fatty acids or n−3 fatty acids, are polyunsaturated fatty acids (PUFAs) characterized by the presence of a double bond, three atoms away from the terminal methyl group in their chemical structure. They are widely distributed in nature, being important constituents of animal lipid metabolism, and they play an important role in the human diet and in human physiology. The three types of omega−3 fatty acids involved in human physiology are α-linolenic acid (ALA), eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). ALA can be found in plants, while DHA and EPA are found in algae and fish. Marine algae and phytoplankton are primary sources of omega−3 fatty acids. DHA and EPA accumulate in fish that eat these algae. Common sources of plant oils containing ALA include walnuts, edible seeds, and flaxseeds as well as hempseed oil, while sources of EPA and DHA include fish and fish oils, and algae oil.
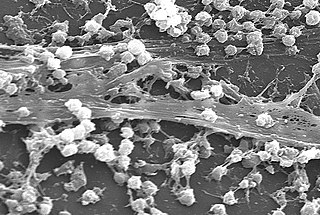
A biofilm comprises any syntrophic consortium of microorganisms in which cells stick to each other and often also to a surface. These adherent cells become embedded within a slimy extracellular matrix that is composed of extracellular polymeric substances (EPSs). The cells within the biofilm produce the EPS components, which are typically a polymeric conglomeration of extracellular polysaccharides, proteins, lipids and DNA. Because they have three-dimensional structure and represent a community lifestyle for microorganisms, they have been metaphorically described as "cities for microbes".

Eicosanoids are signaling molecules made by the enzymatic or non-enzymatic oxidation of arachidonic acid or other polyunsaturated fatty acids (PUFAs) that are, similar to arachidonic acid, around 20 carbon units in length. Eicosanoids are a sub-category of oxylipins, i.e. oxidized fatty acids of diverse carbon units in length, and are distinguished from other oxylipins by their overwhelming importance as cell signaling molecules. Eicosanoids function in diverse physiological systems and pathological processes such as: mounting or inhibiting inflammation, allergy, fever and other immune responses; regulating the abortion of pregnancy and normal childbirth; contributing to the perception of pain; regulating cell growth; controlling blood pressure; and modulating the regional flow of blood to tissues. In performing these roles, eicosanoids most often act as autocrine signaling agents to impact their cells of origin or as paracrine signaling agents to impact cells in the proximity of their cells of origin. Eicosanoids may also act as endocrine agents to control the function of distant cells.

Enoyl-CoA-(∆) isomerase (EC 5.3.3.8, also known as dodecenoyl-CoA- isomerase, 3,2-trans-enoyl-CoA isomerase, ∆3 ,∆2 -enoyl-CoA isomerase, or acetylene-allene isomerase, is an enzyme that catalyzes the conversion of cis- or trans-double bonds of coenzyme A bound fatty acids at gamma-carbon to trans double bonds at beta-carbon as below:
Linoleic acid (LA) is an organic compound with the formula HOOC(CH
2)
7CH=CHCH
2CH=CH(CH
2)
4CH
3. Both alkene groups are cis. It is a fatty acid sometimes denoted 18:2 (n-6) or 18:2 cis-9,12. A linoleate is a salt or ester of this acid.

Conjugated linoleic acids (CLA) are a family of isomers of linoleic acid. In principle, 28 isomers are possible. CLA is found mostly in the meat and dairy products derived from ruminants. The two C=C double bonds are conjugated. CLAs can be either cis-fats or trans-fats.

Docosahexaenoic acid (DHA) is an omega-3 fatty acid that is a primary structural component of the human brain, cerebral cortex, skin, and retina. In physiological literature, it is given the name 22:6(n-3). It can be synthesized from alpha-linolenic acid or obtained directly from maternal milk, fatty fish, fish oil, or algae oil.
In biochemistry and metabolism, beta oxidation (also β-oxidation) is the catabolic process by which fatty acid molecules are broken down in the cytosol in prokaryotes and in the mitochondria in eukaryotes to generate acetyl-CoA. Acetyl-CoA enters the citric acid cycle, generating NADH and FADH2, which are electron carriers used in the electron transport chain. It is named as such because the beta carbon of the fatty acid chain undergoes oxidation and is converted to a carbonyl group to start the cycle all over again. Beta-oxidation is primarily facilitated by the mitochondrial trifunctional protein, an enzyme complex associated with the inner mitochondrial membrane, although very long chain fatty acids are oxidized in peroxisomes.
Dihomo-γ-linolenic acid (DGLA) is a 20-carbon ω−6 fatty acid. In physiological literature, it is given the name 20:3 (ω−6). DGLA is a carboxylic acid with a 20-carbon chain and three cis double bonds; the first double bond is located at the sixth carbon from the omega end. DGLA is the elongation product of γ-linolenic acid. GLA, in turn, is a desaturation product of linoleic acid. DGLA is made in the body by the elongation of GLA, by an efficient enzyme which does not appear to suffer any form of (dietary) inhibition. DGLA is an extremely uncommon fatty acid, found only in trace amounts in animal products.
Eicosatetraenoic acid (ETA) designates any straight chain 20:4 fatty acid. Eicosatetraenoic acid belongs to the family of eicosanoids, molecules synthesized from oxidized polyunsaturated fatty acids (PUFAs) to mediate cell-cell communication. The eicosanoids, working in tandem, contribute to a lipid signaling complex widely responsible for inducing an inflammatory immune response. Common signs of inflammation are both internal and external, with effects like visible redness, pain in the surrounding area, swelling, and the sensation of heat—many of these an effect of varying eicosanoid species. These effects are associated with and have been observed in patients with cancers and various neurological/metabolic disorders.

In biochemistry, glycoside hydrolases are a class of enzymes which catalyze the hydrolysis of glycosidic bonds in complex sugars. They are extremely common enzymes, with roles in nature including degradation of biomass such as cellulose (cellulase), hemicellulose, and starch (amylase), in anti-bacterial defense strategies, in pathogenesis mechanisms and in normal cellular function. Together with glycosyltransferases, glycosidases form the major catalytic machinery for the synthesis and breakage of glycosidic bonds.
Starvation response in animals is a set of adaptive biochemical and physiological changes, triggered by lack of food or extreme weight loss, in which the body seeks to conserve energy by reducing the amount of food energy it consumes.
Vaccenic acid is a naturally occurring trans fatty acid and an omega-7 fatty acid. It is the predominant kind of trans-fatty acid found in human milk, in the fat of ruminants, and in dairy products such as milk, butter, and yogurt. Trans fat in human milk may depend on trans fat content in food.

Stearoyl-CoA desaturase (Δ-9-desaturase) is an endoplasmic reticulum enzyme that catalyzes the rate-limiting step in the formation of monounsaturated fatty acids (MUFAs), specifically oleate and palmitoleate from stearoyl-CoA and palmitoyl-CoA. Oleate and palmitoleate are major components of membrane phospholipids, cholesterol esters and alkyl-diacylglycerol. In humans, the enzyme is encoded by the SCD gene.
Biofilm formation occurs when free floating microorganisms attach themselves to a surface. Although there are some beneficial uses of biofilms, they are generally considered undesirable, and means of biofilm prevention have been developed. Biofilms secrete extracellular polymeric substance that provides a structural matrix and facilitates adhesion for the microorganisms; the means of prevention have thus concentrated largely on two areas: killing the microbes that form the film, or preventing the adhesion of the microbes to a surface. Because biofilms protect the bacteria, they are often more resistant to traditional antimicrobial treatments, making them a serious health risk. For example, there are more than one million cases of catheter-associated urinary tract infections (CAUTI) reported each year, many of which can be attributed to bacterial biofilms. There is much research into the prevention of biofilms.
Decenoic acid is any mono-carboxylic acid with an unbranched chain of ten carbons connected by eight single bonds and one double bond; that is, a chemical compound with formula HO(O=)C–(CH
2)
k–CH=CH–(CH
2)
7-k–H, where k is between 0 and 7 inclusive.

Coronaric acid (leukotoxin or leukotoxin A) is a mono-unsaturated, epoxide derivative of the di-saturated fatty acid, linoleic acid (i.e. 9(Z),12(Z) octadecadienoic acid). It is a mixture of the two optically active isomers of 12(Z) 9,10-epoxy-octadecenoic acid. This mixture is also termed 9,10-epoxy-12Z-octadecenoic acid or 9(10)-EpOME and when formed by or studied in mammalians, leukotoxin.
Diffusible signal factor (DSF) is found in several gram-negative bacteria and play a role in the formation of biofilms, motility, virulence, and antibiotic resistance. Xanthomonas campestris was the first bacteria known to have DSF. The synthesis of the DSF can be seen in rpfF and rpfB enzymes. An understanding of the DSF signaling mechanism could lead to further disease control.











