Aromatic compounds or arenes usually refers to organic compounds "with a chemistry typified by benzene" and "cyclically conjugated." The word "aromatic" originates from the past grouping of molecules based on odor, before their general chemical properties were understood. The current definition of aromatic compounds does not have any relation to their odor. Aromatic compounds are now defined as cyclic compounds satisfying Hückel's Rule. Aromatic compounds have the following general properties:

Naphthalene is an organic compound with formula C
10H
8. It is the simplest polycyclic aromatic hydrocarbon, and is a white crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 ppm by mass. As an aromatic hydrocarbon, naphthalene's structure consists of a fused pair of benzene rings. It is the main ingredient of traditional mothballs.

In theoretical chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in a molecule, which in general lowers the overall energy of the molecule and increases stability. It is conventionally represented as having alternating single and multiple bonds. Lone pairs, radicals or carbenium ions may be part of the system, which may be cyclic, acyclic, linear or mixed. The term "conjugated" was coined in 1899 by the German chemist Johannes Thiele.

In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected by the stabilization of conjugation alone. The earliest use of the term was in an article by August Wilhelm Hofmann in 1855. There is no general relationship between aromaticity as a chemical property and the olfactory properties of such compounds.
In chemistry, resonance, also called mesomerism, is a way of describing bonding in certain molecules or polyatomic ions by the combination of several contributing structures into a resonance hybrid in valence bond theory. It has particular value for analyzing delocalized electrons where the bonding cannot be expressed by one single Lewis structure. The resonance hybrid is the accurate structure for a molecule or ion; it is an average of the theoretical contributing structures.

Anthracene is a solid polycyclic aromatic hydrocarbon (PAH) of formula C14H10, consisting of three fused benzene rings. It is a component of coal tar. Anthracene is used in the production of the red dye alizarin and other dyes. Anthracene is colorless but exhibits a blue (400–500 nm peak) fluorescence under ultraviolet radiation.

Phenanthrene is a polycyclic aromatic hydrocarbon (PAH) with formula C14H10, consisting of three fused benzene rings. It is a colorless, crystal-like solid, but can also appear yellow. Phenanthrene is used to make dyes, plastics, pesticides, explosives, and drugs. It has also been used to make bile acids, cholesterol and steroids.

Coronene is a polycyclic aromatic hydrocarbon (PAH) comprising seven peri-fused benzene rings. Its chemical formula is C
24H
12. It is a yellow material that dissolves in common solvents including benzene, toluene, and dichloromethane. Its solutions emit blue light fluorescence under UV light. It has been used as a solvent probe, similar to pyrene.

A polycyclic aromatic hydrocarbon (PAH) is a class of organic compounds that is composed of multiple aromatic rings. The simplest representative is naphthalene, having two aromatic rings, and the three-ring compounds anthracene and phenanthrene. PAHs are uncharged, non-polar and planar. Many are colorless. Many of them are found in coal and in oil deposits, and are also produced by the incomplete combustion of organic matter—for example, in engines and incinerators or when biomass burns in forest fires.
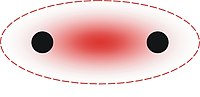
In organic chemistry, Hückel's rule predicts that a planar ring molecule will have aromatic properties if it has 4n + 2 π electrons, where n is a non-negative integer. The quantum mechanical basis for its formulation was first worked out by physical chemist Erich Hückel in 1931. The succinct expression as the 4n + 2 rule has been attributed to W. v. E. Doering (1951), although several authors were using this form at around the same time.
Antiaromaticity is a chemical property of a cyclic molecule with a π electron system that has higher energy, i.e., it is less stable due to the presence of 4n delocalised electrons in it, as opposed to aromaticity. Unlike aromatic compounds, which follow Hückel's rule and are highly stable, antiaromatic compounds are highly unstable and highly reactive. To avoid the instability of antiaromaticity, molecules may change shape, becoming non-planar and therefore breaking some of the π interactions. In contrast to the diamagnetic ring current present in aromatic compounds, antiaromatic compounds have a paramagnetic ring current, which can be observed by NMR spectroscopy.
Simple aromatic rings, also known as simple arenes or simple aromatics, are aromatic organic compounds that consist only of a conjugated planar ring system. Many simple aromatic rings have trivial names. They are usually found as substructures of more complex molecules. Typical simple aromatic compounds are benzene, indole, and pyridine.

Triphenylene is an organic compound with the formula (C6H4)3. A flat polycyclic aromatic hydrocarbon (PAH), it consists of four fused benzene rings. Triphenylene has delocalized 18-π-electron systems based on a planar structure, corresponding to the symmetry group D3h. It is a white or colorless solid.
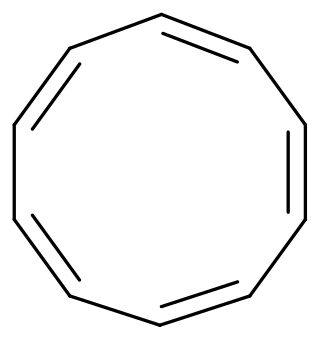
Cyclodecapentaene or [10]annulene is an annulene with molecular formula C10H10. This organic compound is a conjugated 10 pi electron cyclic system and according to Huckel's rule it should display aromaticity. It is not aromatic, however, because various types of ring strain destabilize an all-planar geometry.

A cyclic compound is a term for a compound in the field of chemistry in which one or more series of atoms in the compound is connected to form a ring. Rings may vary in size from three to many atoms, and include examples where all the atoms are carbon, none of the atoms are carbon, or where both carbon and non-carbon atoms are present. Depending on the ring size, the bond order of the individual links between ring atoms, and their arrangements within the rings, carbocyclic and heterocyclic compounds may be aromatic or non-aromatic; in the latter case, they may vary from being fully saturated to having varying numbers of multiple bonds between the ring atoms. Because of the tremendous diversity allowed, in combination, by the valences of common atoms and their ability to form rings, the number of possible cyclic structures, even of small size numbers in the many billions.

An aromatic ring current is an effect observed in aromatic molecules such as benzene and naphthalene. If a magnetic field is directed perpendicular to the plane of the aromatic system, a ring current is induced in the delocalized π electrons of the aromatic ring. This is a direct consequence of Ampère's law; since the electrons involved are free to circulate, rather than being localized in bonds as they would be in most non-aromatic molecules, they respond much more strongly to the magnetic field.
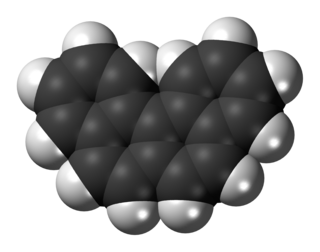
Benzo[c]phenanthrene is a polycyclic aromatic hydrocarbon with the chemical formula C18H12. It is a white solid that is soluble in nonpolar organic solvents. It is a nonplanar molecule consisting of the fusion of four fused benzene rings. The compound is of mainly theoretical interest but it is environmentally occurring and weakly carcinogenic.
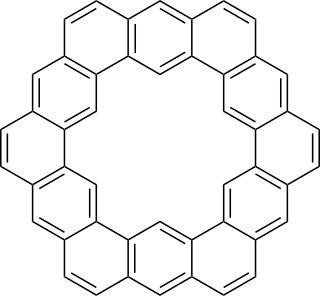
Kekulene is a polycyclic aromatic hydrocarbon which consists of 12 fused benzene rings arranged in a circle. It is therefore classified as a [12]-circulene with the chemical formula C48H24. It was first synthesized in 1978, and was named in honor of August Kekulé, the discoverer of the structure of the benzene molecule.
In chemistry, a C–H···O interaction is occasionally described as a special type of weak hydrogen bond. These interactions frequently occur in the structures of important biomolecules like amino acids, proteins, sugars, DNA and RNA.

Boraacenes are polycyclic aromatic hydrocarbons containing at least one boron atom. Structurally, they are related to acenes, linearly fused benzene rings. However, the boron atom is electron deficient and may act as a Lewis Acid when compared to carbon. This results in slightly less negative charge within the ring, smaller HOMO-LUMO gaps, as well as differences in redox chemistry when compared to their acene analogues. When incorporated into acenes, Boron maintains the planarity and aromaticity of carbon acenes, while adding an empty p-orbital, which can be utilized for the fine tuning of organic semiconductor band gaps. Due to this empty p orbital, however, it is also highly reactive when exposed to nucleophiles like water or normal atmosphere, as it will readily be attacked by oxygen, which must be addressed to maintain its stability.