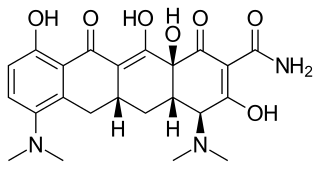
Minocycline, sold under the brand name Minocin among others, is a tetracycline antibiotic medication used to treat a number of bacterial infections such as some occurring in certain forms of pneumonia. It is generally less preferred than the tetracycline doxycycline. Minocycline is also used for the treatment of acne and rheumatoid arthritis. It is taken by mouth or applied to the skin.

Immunosuppressive drugs, also known as immunosuppressive agents, immunosuppressants and antirejection medications, are drugs that inhibit or prevent the activity of the immune system.

Mycobacterium is a genus of over 190 species in the phylum Actinomycetota, assigned its own family, Mycobacteriaceae. This genus includes pathogens known to cause serious diseases in mammals, including tuberculosis and leprosy in humans. The Greek prefix myco- means 'fungus', alluding to this genus' mold-like colony surfaces. Since this genus has cell walls with a waxy lipid-rich outer layer containing high concentrations of mycolic acid, acid-fast staining is used to emphasize their resistance to acids, compared to other cell types.
Mycobacterium leprae is one of the two species of bacteria that cause Hansen's disease (leprosy), a chronic but curable infectious disease that damages the peripheral nerves and targets the skin, eyes, nose, and muscles.
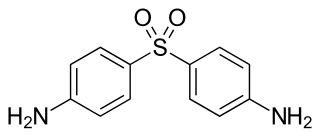
Dapsone, also known as 4,4'-sulfonyldianiline (SDA) or diaminodiphenyl sulfone (DDS), is an antibiotic commonly used in combination with rifampicin and clofazimine for the treatment of leprosy. It is a second-line medication for the treatment and prevention of pneumocystis pneumonia and for the prevention of toxoplasmosis in those who have poor immune function. Additionally, it has been used for acne, dermatitis herpetiformis, and various other skin conditions. Dapsone is available both topically and by mouth.

Pimecrolimus is an immunosuppressant drug of the calcineurin inhibitor class used in the treatment of atopic dermatitis (eczema).

Rifampicin, also known as rifampin, is an ansamycin antibiotic used to treat several types of bacterial infections, including tuberculosis (TB), Mycobacterium avium complex, leprosy, and Legionnaires' disease. It is almost always used together with other antibiotics with two notable exceptions: when given as a "preferred treatment that is strongly recommended" for latent TB infection; and when used as post-exposure prophylaxis to prevent Haemophilus influenzae type b and meningococcal disease in people who have been exposed to those bacteria. Before treating a person for a long period of time, measurements of liver enzymes and blood counts are recommended. Rifampicin may be given either by mouth or intravenously.

Mepacrine, also called quinacrine or by the trade names Atabrine or Atebrin, is a medication with several uses. It is related to chloroquine and mefloquine. Although available from compounding pharmacies, as of August 2020 approved formulations are not available in the United States.

Penicillamine, sold under the brand name of Cuprimine among others, is a medication primarily used for the treatment of Wilson's disease. It is also used for people with kidney stones who have high urine cystine levels, rheumatoid arthritis, and various heavy metal poisonings. It is taken by mouth.

Lupus vulgaris are painful cutaneous tuberculosis skin lesions with nodular appearance, most often on the face around the nose, eyelids, lips, cheeks, ears and neck. It is the most common Mycobacterium tuberculosis skin infection. The lesions may ultimately develop into disfiguring skin ulcers if left untreated.

Mycobacterium avium-intracellulare infection (MAI) is an atypical mycobacterial infection, i.e. one with nontuberculous mycobacteria or NTM, caused by Mycobacterium avium complex (MAC), which is made of two Mycobacterium species, M. avium and M. intracellulare. This infection causes respiratory illness in birds, pigs, and humans, especially in immunocompromised people. In the later stages of AIDS, it can be very severe. It usually first presents as a persistent cough. It is typically treated with a series of three antibiotics for a period of at least six months.

Belimumab, sold under the brand name Benlysta, is a human monoclonal antibody that inhibits B-cell activating factor (BAFF), also known as B-lymphocyte stimulator (BLyS). It is approved in the United States and Canada, and the European Union to treat systemic lupus erythematosus and lupus nephritis.

Potassium voltage-gated channel, shaker-related subfamily, member 3, also known as KCNA3 or Kv1.3, is a protein that in humans is encoded by the KCNA3 gene.

Lupus erythematosus is a collection of autoimmune diseases in which the human immune system becomes hyperactive and attacks healthy tissues. Symptoms of these diseases can affect many different body systems, including joints, skin, kidneys, blood cells, heart, and lungs. The most common and most severe form is systemic lupus erythematosus.
A leprostatic agent is a drug that interferes with proliferation of the bacterium that causes leprosy.
Tumid lupus erythematosus is a rare, but distinctive entity in which patients present with edematous erythematous plaque.
Palisaded neutrophilic and granulomatous dermatitis (PNGS) is usually associated with a well-defined connective tissue disease, lupus erythematosus or rheumatoid arthritis most commonly, and often presents with eroded or ulcerated symmetrically distributed umbilicated papules or nodules on the elbows.

Lupus, formally called systemic lupus erythematosus (SLE), is an autoimmune disease in which the body's immune system mistakenly attacks healthy tissue in many parts of the body. Symptoms vary among people and may be mild to severe. Common symptoms include painful and swollen joints, fever, chest pain, hair loss, mouth ulcers, swollen lymph nodes, feeling tired, and a red rash which is most commonly on the face. Often there are periods of illness, called flares, and periods of remission during which there are few symptoms. Children up to 18 years old develop a more severe form of SLE termed childhood-onset systemic lupus erythematosus.
Blisibimod is a selective antagonist of B-cell activating factor, being developed by Anthera Pharmaceuticals as a treatment for systemic lupus erythematosus. It is currently under active investigation in clinical trials.

Promin, or sodium glucosulfone is a sulfone drug that was investigated for the treatment of malaria, tuberculosis and leprosy. It is broken down in the body to dapsone, which is the therapeutic form.

















