Related Research Articles

Polyoxybenzylmethylenglycolanhydride, better known as Bakelite, was the first plastic made from synthetic components. It is a thermosetting phenol formaldehyde resin, formed from a condensation reaction of phenol with formaldehyde. It was developed by the Belgian chemist Leo Baekeland in Yonkers, New York, in 1907.

Imperial Chemical Industries (ICI) was a British chemical company. It was, for much of its history, the largest manufacturer in Britain. It was formed by the merger of four leading British chemical companies in 1926. Its headquarters were at Millbank in London. ICI was a constituent of the FT 30 and later the FTSE 100 indices.

The chemical industry comprises the companies that produce industrial chemicals. Central to the modern world economy, it converts raw materials into more than 70,000 different products. The plastics industry contains some overlap, as some chemical companies produce plastics as well as chemicals.

Petrochemicals are the chemical products obtained from petroleum by refining. Some chemical compounds made from petroleum are also obtained from other fossil fuels, such as coal or natural gas, or renewable sources such as maize, palm fruit or sugar cane.

Sodium carbonate, Na2CO3·10H2O, (also known as washing soda, soda ash and soda crystals) is the inorganic compound with the formula Na2CO3 and its various hydrates. All forms are white, odourless, water-soluble salts that yield moderately alkaline solutions in water. Historically, it was extracted from the ashes of plants growing in sodium-rich soils. Because the ashes of these sodium-rich plants were noticeably different from ashes of wood (once used to produce potash), sodium carbonate became known as "soda ash". It is produced in large quantities from sodium chloride and limestone by the Solvay process.
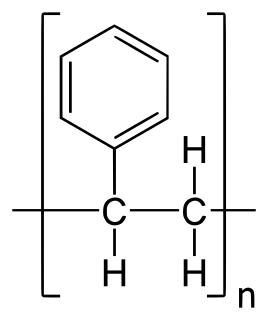
Polystyrene (PS) is a synthetic aromatic hydrocarbon polymer made from the monomer known as styrene. Polystyrene can be solid or foamed. General-purpose polystyrene is clear, hard, and brittle. It is an inexpensive resin per unit weight. It is a poor barrier to oxygen and water vapour and has a relatively low melting point. Polystyrene is one of the most widely used plastics, the scale of its production being several million tonnes per year. Polystyrene can be naturally transparent, but can be coloured with colourants. Uses include protective packaging, containers, lids, bottles, trays, tumblers, disposable cutlery, in the making of models, and as an alternative material for phonograph records.

BASF SE is a German multinational chemical company and the largest chemical producer in the world. The BASF Group comprises subsidiaries and joint ventures in more than 80 countries and operates six integrated production sites and 390 other production sites in Europe, Asia, Australia, the Americas and Africa. Its headquarters is located in Ludwigshafen, Germany. BASF has customers in over 190 countries and supplies products to a wide variety of industries. Despite its size and global presence, BASF has received relatively little public attention since it abandoned manufacturing and selling BASF-branded consumer electronics products in the 1990s.

A thermoplastic, or thermosoft plastic, is a plastic polymer material that becomes pliable or moldable at a certain elevated temperature and solidifies upon cooling.

Acrylonitrile butadiene styrene (ABS) (chemical formula (C8H8)x·(C4H6)y·(C3H3N)z) is a common thermoplastic polymer. Its glass transition temperature is approximately 220 °F (104 °C). ABS is amorphous and therefore has no true melting point.

Polycarbonates (PC) are a group of thermoplastic polymers containing carbonate groups in their chemical structures. Polycarbonates used in engineering are strong, tough materials, and some grades are optically transparent. They are easily worked, molded, and thermoformed. Because of these properties, polycarbonates find many applications. Polycarbonates do not have a unique resin identification code (RIC) and are identified as "Other", 7 on the RIC list. Products made from polycarbonate can contain the precursor monomer bisphenol A (BPA).

PPG Industries, Inc. is an American Fortune 500 company and global supplier of paints, coatings, and specialty materials. With headquarters in Pittsburgh, Pennsylvania, PPG operates in more than 70 countries around the globe. By revenue it is the largest coatings company in the world followed by AkzoNobel. It is headquartered in PPG Place, an office and retail complex in downtown Pittsburgh, and is known for its glass facade designed by Philip Johnson.
Dehydrogenation is the chemical reaction that involves the removal of hydrogen, usually from an organic molecule. It is the reverse of hydrogenation. Dehydrogenation is important, both as a useful reaction and a serious problem. At its simplest, it is useful way of converting alkanes, which are relatively inert and thus low-valued, to olefins, which are reactive and thus more valuable. Alkenes are precursors to aldehydes, alcohols, polymers, and aromatics. As a problematic reaction, the fouling and inactivation of many catalysts arises via coking, which is the dehydrogenative polymerization of organic substrates.

Formamide is an amide derived from formic acid. It is a colorless liquid which is miscible with water and has an ammonia-like odor. It is chemical feedstock for the manufacture of sulfa drugs, other pharmaceuticals, herbicides, pesticides and the manufacture of hydrocyanic acid. It has been used as a softener for paper and fiber. It is a solvent for many ionic compounds. It has also been used as a solvent for resins and plasticizers.

Plastic recycling is the reprocessing of plastic waste into new and useful products. When performed correctly, this can reduce dependence on landfill, conserve resources and protect the environment from plastic pollution and greenhouse gas emissions. Although recycling rates are increasing, they lag behind those of other recoverable materials, such as aluminium, glass and paper. The global recycling rate in 2015 was 19.5%, while 25.5% was incinerated and the remaining 55% disposed of to landfill. Since the beginning of plastic production in the 20th century, until 2015, the world has produced some 6.3 billion tonnes of plastic waste, only 9% of which has been recycled, and only ~1% has been recycled more than once.

A static mixer is a precision engineered device for the continuous mixing of fluid materials, without moving components. Normally the fluids to be mixed are liquid, but static mixers can also be used to mix gas streams, disperse gas into liquid or blend immiscible liquids. The energy needed for mixing comes from a loss in pressure as fluids flow through the static mixer. One design of static mixer is the plate-type mixer and another common device type consists of mixer elements contained in a cylindrical (tube) or squared housing. Mixer size can vary from about 6 mm to 6 meters diameter. Typical construction materials for static mixer components include stainless steel, polypropylene, Teflon, PVDF, PVC, CPVC and polyacetal. The latest designs involve static mixing elements made of glass-lined steel.

CR-39, or allyl diglycol carbonate (ADC), is a plastic monomer commonly used in the manufacture of eyeglass lenses alongside the other material PMMA.

Koppers is a global chemical and materials company based in Pittsburgh, Pennsylvania, United States in an art-deco 1920s skyscraper, the Koppers Tower.

A carbonate ester (organic carbonate or organocarbonate) is an ester of carbonic acid. This functional group consists of a carbonyl group flanked by two alkoxy groups. The general structure of these carbonates is R1O(C=O)OR2 and they are related to esters R1O(C=O)R, ethers R1OR2 and also to the inorganic carbonates.

The University of Pittsburgh Applied Research Center (U-PARC) is a one-million-square foot, high-security research park campus of the University of Pittsburgh. Comprising 53 buildings situated on over 85 acres (0.34 km2), U-PARC is located 14 miles (23 km) from Downtown Pittsburgh in Harmar Township, Pennsylvania adjacent to the Route 28 expressway and Interstate 76, the Pennsylvania Turnpike.

Plastics are a wide range of synthetic or semi-synthetic materials that use polymers as a main ingredient. Their plasticity makes it possible for plastics to be moulded, extruded or pressed into solid objects of various shapes. This adaptability, plus a wide range of other properties, such as being lightweight, durable, flexible, and inexpensive to produce, has led to its widespread use. Plastics typically are made through human industrial systems. Most modern plastics are derived from fossil fuel-based chemicals like natural gas or petroleum; however, recent industrial methods use variants made from renewable materials, such as corn or cotton derivatives.
References
- ↑ Collected Reprints of McBee's Annual Reviews of Halogenation, 1948–1958: Reprinted from Industrial and Engineering Chemistry for Columbia-Southern Chemical Corporation. 1950. p. 2082.
- ↑ Gribbin, John H.; Krogfus, Sue Singer (1960). Industrial Research Laboratories of the United States (11 ed.). Washington, D.C.: National Academy of Sciences—National Research Council. p. 117 #884–891, p. 374 #2984. LCCN 21-26022.
- ↑ "Progress at Pittsburgh Plate – In CHemical Operations". Pittsburgh Post-Gazette. January 4, 1956. p. 20. Retrieved January 29, 2016.
- 1 2 "Columbia-Southern Chemical Corporation". The Cornell Daily Sun. Ithaca, New York. February 15, 1960. p. 5. Retrieved January 29, 2016.
- ↑ Hempstead, Colin A., ed. (2004). Encyclopedia of 20th-Century Technology. Vol. 2 M-Z. New York and London: Routledge. p. 563, Optometry – Materials. ISBN 1579584640.
- ↑ "Plastic – Materials used for Plastic Lenses". Where does the name CR 39 come from?. Carl Zeiss Vision Inc. Retrieved January 28, 2016.
- ↑ "Ammonia Unit for Natrium". Pittsburgh Post-Gazette. August 12, 1953. p. 19. Retrieved January 29, 2016.
- ↑ "Chemical Firm Extends Program". Pittsburgh Post-Gazette. May 1, 1952. p. 21. Retrieved January 29, 2016.
- ↑ Mauriello, Tracie (November 1, 2015). "Pittsburgh companies part of $7 billion claim against Cuba". Pittsburgh Post-Gazette. Washington Bureau. Retrieved January 29, 2016.
