The complement system, also known as complement cascade, is a part of the immune system that enhances (complements) the ability of antibodies and phagocytic cells to clear microbes and damaged cells from an organism, promote inflammation, and attack the pathogen's cell membrane. It is part of the innate immune system, which is not adaptable and does not change during an individual's lifetime. The complement system can, however, be recruited and brought into action by antibodies generated by the adaptive immune system.

Perforin-1 is a protein that in humans is encoded by the PRF1 gene and the Prf1 gene in mice.

The classical complement pathway is one of three pathways which activate the complement system, which is part of the immune system. The classical complement pathway is initiated by antigen-antibody complexes with the antibody isotypes IgG and IgM.

C1-inhibitor is a protease inhibitor belonging to the serpin superfamily. Its main function is the inhibition of the complement system to prevent spontaneous activation but also as the major regulator of the contact system. C1-inhibitor is an acute-phase protein that circulates in blood at levels of around 0.25 g/L. The levels rise ~2-fold during inflammation. C1-inhibitor irreversibly binds to and inactivates C1r and C1s proteases in the C1 complex of classical pathway of complement. MASP-1 and MASP-2 proteases in MBL complexes of the lectin pathway are also inactivated. This way, C1-inhibitor prevents the proteolytic cleavage of later complement components C4 and C2 by C1 and MBL. Although named after its complement inhibitory activity, C1-inhibitor also inhibits proteases of the fibrinolytic, clotting, and kinin pathways. Note that C1-inhibitor is the most important physiological inhibitor of plasma kallikrein, FXIa, and FXIIa.

Thrombomodulin (TM), CD141 or BDCA-3 is an integral membrane protein expressed on the surface of endothelial cells and serves as a cofactor for thrombin. It reduces blood coagulation by converting thrombin to an anticoagulant enzyme from a procoagulant enzyme. Thrombomodulin is also expressed on human mesothelial cell, monocyte and a dendritic cell subset.

The lectin pathway or MBL pathway is a type of cascade reaction in the complement system, similar in structure to the classical complement pathway, in that, after activation, it proceeds through the action of C4 and C2 to produce activated complement proteins further down the cascade. In contrast to the classical complement pathway, the lectin pathway does not recognize an antibody bound to its target. The lectin pathway starts with mannose-binding lectin (MBL) or ficolin binding to certain sugars.

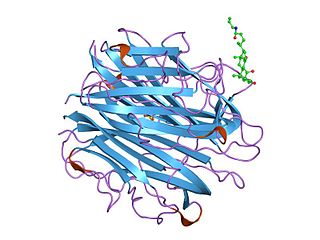
Complement C2 is a protein that in humans is encoded by the C2 gene. The protein encoded by this gene is part of the classical pathway of the complement system, acting as a multi-domain serine protease. Deficiency of C2 has been associated with certain autoimmune diseases.

Complement component 5 is a protein that in humans is encoded by the C5 gene.

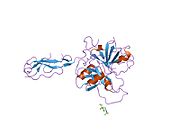
Complement component 1s is a protein involved in the complement system. C1s is part of the C1 complex. In humans, it is encoded by the C1S gene.

Mannan-binding lectin serine protease 1 also known as mannose-associated serine protease 1 (MASP-1) is an enzyme that in humans is encoded by the MASP1 gene.

Mannan-binding lectin serine protease 2 also known as mannose-binding protein-associated serine protease 2 (MASP-2) is an enzyme that in humans is encoded by the MASP2 gene.
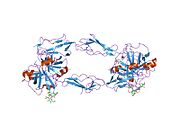
The complement component 1q is a protein complex involved in the complement system, which is part of the innate immune system. C1q together with C1r and C1s form the C1 complex.

Complement C1q subcomponent subunit A is a protein that in humans is encoded by the C1QA gene.

The EGF-like domain is an evolutionary conserved protein domain, which derives its name from the epidermal growth factor where it was first described. It comprises about 30 to 40 amino-acid residues and has been found in a large number of mostly animal proteins. Most occurrences of the EGF-like domain are found in the extracellular domain of membrane-bound proteins or in proteins known to be secreted. An exception to this is the prostaglandin-endoperoxide synthase. The EGF-like domain includes 6 cysteine residues which in the epidermal growth factor have been shown to form 3 disulfide bonds. The structures of 4-disulfide EGF-domains have been solved from the laminin and integrin proteins. The main structure of EGF-like domains is a two-stranded β-sheet followed by a loop to a short C-terminal, two-stranded β-sheet. These two β-sheets are usually denoted as the major (N-terminal) and minor (C-terminal) sheets. EGF-like domains frequently occur in numerous tandem copies in proteins: these repeats typically fold together to form a single, linear solenoid domain block as a functional unit.

Complement component 1 Q subcomponent-binding protein, mitochondrial is a protein that in humans is encoded by the C1QBP gene.

C3a is one of the proteins formed by the cleavage of complement component 3; the other is C3b. C3a is a 77 residue anaphylatoxin that binds to the C3a receptor (C3aR), a class A G protein-coupled receptor. It plays a large role in the immune response.
CUB domain is an evolutionarily conserved protein domain. The CUB domain is a structural motif of approximately 110 residues found almost exclusively in extracellular and plasma membrane-associated proteins, many of which are developmentally regulated. These proteins are involved in a diverse range of functions, including complement activation, developmental patterning, tissue repair, axon guidance and angiogenesis, cell signalling, fertilisation, haemostasis, inflammation, neurotransmission, receptor-mediated endocytosis, and tumour suppression. Many CUB-containing proteins are peptidases belonging to MEROPS peptidase families M12A (astacin) and S1A (chymotrypsin).

The complement component 1, q subcomponent-like 1 is encoded by a gene located at chromosome 17q21.31. It is a secreted protein and is 258 amino acids in length. The protein is widely expressed but its expression is highest in the brain and may also be involved in regulation of motor control. The pre-mRNA of this protein is subject to RNA editing.
Mannan-binding lectin-associated serine protease-2 is an enzyme. This enzyme catalyses the following chemical reaction
The C1 complex is a protein complex involved in the complement system. It is the first component of the classical complement pathway and is composed of the subcomponents C1q, C1r and C1s.