
Apiaceae or Umbelliferae is a family of mostly aromatic flowering plants named after the type genus Apium and commonly known as the celery, carrot or parsley family, or simply as umbellifers. It is the 16th-largest family of flowering plants, with more than 3,800 species in about 446 genera, including such well-known and economically important plants as ajwain, angelica, anise, asafoetida, caraway, carrot, celery, chervil, coriander, cumin, dill, fennel, lovage, cow parsley, parsley, parsnip and sea holly, as well as silphium, a plant whose exact identity is unclear and which may be extinct.

Daucus carota, whose common names include wild carrot, European wild carrot, bird's nest, bishop's lace, and Queen Anne's lace, is a flowering plant in the family Apiaceae. It is native to temperate regions of the Old World and was naturalized in the New World.

Oenanthe, known as water dropworts, oenanthes, water parsleys, and water celeries, are a genus of plants in the family Apiaceae. Most of the species grow in damp ground, such as in marshes or in water.

Coniine is a poisonous chemical compound, an alkaloid present in and isolable from poison hemlock (Conium maculatum), where its presence has been a source of significant economic, medical, and historico-cultural interest; coniine is also produced by the yellow pitcher plant (Sarracenia flava), and fool's parsley (Aethusa cynapium). Its ingestion and extended exposure are toxic to humans and all classes of livestock; its mechanism of poisoning involves disruption of the central nervous system, with death caused by respiratory paralysis. The biosynthesis of coniine contains as its penultimate step the non-enzymatic cyclisation of 5-oxooctylamine to γ-coniceine, a Schiff base differing from coniine only by its carbon-nitrogen double bond in the ring. This pathway results in natural coniine that is a mixture—a racemate—composed of two enantiomers, the stereoisomers (S)-(+)-coniine and (R)-(−)-coniine, depending on the direction taken by the chain that branches from the ring. Both enantiomers are toxic, with the (R)-enantiomer being the more biologically active and toxic of the two in general. Coniine holds a place in organic chemistry history as being the first of the important class of alkaloids to be synthesized, by Albert Ladenburg in 1886, and it has been synthesized in the laboratory in a number of unique ways through to modern times.

Cicuta virosa, the cowbane or northern water hemlock, is a poisonous species of Cicuta, native to northern and central Europe, northern Asia and northwestern North America.
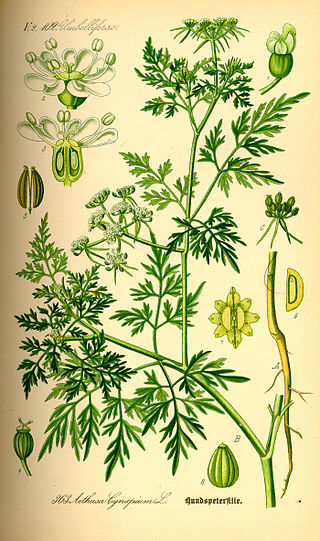
Aethusa cynapium is an annual herb in the flowering plant family Apiaceae, native to Europe, western Asia, and northwest Africa. It is the only member of the genus Aethusa. It is related to hemlock and water-dropwort, and like them it is poisonous, though less so than hemlock. It has been introduced into many other parts of the world and is a common weed in cultivated ground.

Cicutoxin is a naturally-occurring poisonous chemical compound produced by several plants from the family Apiaceae including water hemlock (Cicuta species) and water dropwort (Oenanthe crocata). The compound contains polyene, polyyne, and alcohol functional groups and is a structural isomer of oenanthotoxin, also found in water dropwort. Both of these belong to the C17-polyacetylenes chemical class.

Cicuta, commonly known as water hemlock, is a genus of four species of highly poisonous plants in the family Apiaceae. They are perennial herbaceous plants which grow up to 2.5 meters (8 ft) tall, having distinctive small green or white flowers arranged in an umbrella shape (umbel). Plants in this genus may also be referred to as cowbane or poison parsnip. Cicuta is native to temperate regions of the Northern Hemisphere, mainly North America and Europe, typically growing in wet meadows, along streambanks and other wet and marshy areas. These plants bear a close resemblance to other members in the family Apiaceae and may be confused with a number of edible or poisonous plants. The common name hemlock may also be confused with poison hemlock, or with the Hemlock tree.

Cicuta maculata is a highly poisonous species of flowering plant in the carrot family known by several common names, including spotted water hemlock, spotted parsley, spotted cowbane, and the suicide root by the Iroquois. It is native to nearly all of North America, from northern Canada to southern Mexico.

Ligusticum porteri, also known as oshá, wild parsnip, Porter’s Lovage or wild celery, is a perennial herb found in parts of the Rocky Mountains and northern New Mexico, especially in the southwestern United States.

Berula is a cosmopolitan genus of flowering plants in the family Apiaceae, whose species are known as water parsnips, as are some other plants in Apiaceae such as Sium latifolium and Sium suave. It is easily confused with the highly toxic water hemlock.

Sium suave, the water parsnip or hemlock waterparsnip, is a perennial wildflower in the family Apiaceae. It is native to many areas of both Asia and North America. The common name water parsnip is due to its similarity to parsnip and its wetland habitat. The alternate common name hemlock waterparsnip is due to its similarity to the highly poisonous spotted water hemlock.

Coturnism is an illness featuring muscle tenderness and rhabdomyolysis after consuming quail that have fed on poisonous plants.

The hemlock moth, also known as the defoliating hemlock moth or poison hemlock moth, is a nocturnal moth species of the family Depressariidae. Of Palaearctic origin, it was first found in North America in 1973 when it was accidentally introduced. The moth is now widespread throughout the northern half of the United States, southern Canada, northern Europe, and, more recently, New Zealand and Australia. The larval form grows to around 10 mm, while the adults wingspan is between 17 mm and 19 mm.
Apium virus Y (ApVY) is a plant pathogenic virus in the genus Potyvirus and the virus family Potyviridae.

Conium maculatum, colloquially known as hemlock, poison hemlock or wild hemlock, is a highly poisonous flowering plant in the carrot family Apiaceae, native to Europe and North Africa. It is herbaceous without woody parts and has a biennial lifecycle. A hardy plant capable of living in a variety of environments, hemlock is widely naturalised in locations outside its native range, such as parts of Australia, West Asia, and North and South America, to which it has been introduced. It is capable of spreading and thereby becoming an invasive weed.

Cicuta bulbifera, commonly known as the bulb-bearing water-hemlock, is a plant native to North America and one of four species in the poisonous genus Cicuta. Tiny bulbils form in the leaf joints in the upper part of the plant, giving the plant its scientific and common names. Cicuta bulbifera can be distinguished from Cicuta douglasii by its narrow leaflet segments and its bulbil-bearing upper leaf axils.

Oenanthe crocata, hemlock water-dropwort is a flowering plant in the carrot family, native to Europe, North Africa and western Asia. It grows in damp grassland and wet woodland, often along river and stream banks. All parts of the plant are extremely toxic and it has been known to cause human and livestock poisoning.


















