Related Research Articles

The term narcotic originally referred medically to any psychoactive compound with numbing or paralyzing properties. In the United States, it has since become associated with opiates and opioids, commonly morphine and heroin, as well as derivatives of many of the compounds found within raw opium latex. The primary three are morphine, codeine, and thebaine.

Dihydrocodeine is a semi-synthetic opioid analgesic prescribed for pain or severe dyspnea, or as an antitussive, either alone or compounded with paracetamol (acetaminophen) or aspirin. It was developed in Germany in 1908 and first marketed in 1911.

The Single Convention on Narcotic Drugs, 1961 is an international treaty that controls activities of specific narcotic drugs and lays down a system of regulations for their medical and scientific uses; it also establishes the International Narcotics Control Board.
The International Narcotics Control Board (INCB) is an independent treaty body, one of the four treaty-mandated bodies under international drug control law.
The expression International Opium Convention refers either to the first International Opium Convention signed at The Hague in 1912, or to the second International Opium Convention signed at Geneva in 1925.
The Misuse of Drugs Act 1977, the Misuse of Drugs Act 1984, Misuse of Drugs Act 2015 and the Criminal Justice Act 2010 are the acts of the Oireachtas regulating drugs in Ireland. The acts define the penalties for unlawful production, possession and supply of drugs.
The Protocol bringing under International Control Drugs Outside of the Scope of the 1931 Convention for Limiting the Manufacture and Regulating the Distribution of Narcotic Drugs, signed in 1948, was a drug control treaty designed to eliminate some of the loopholes associate with its predecessor treaty, the 1931 Convention for Limiting the Manufacture and Regulating the Distribution of Narcotic Drugs. It is perhaps most noteworthy as the first drug control treaty to implement the similarity concept; that is, its provisions applied to all drugs with similar harmful effects and abuse liabilities as the drugs specified in article I, paragraph 2 of the 1931 treaty Archived October 12, 2007, at the Wayback Machine. The similarity concept was also included in the 1961 Single Convention on Narcotic Drugs and the 1971 Convention on Psychotropic Substances.
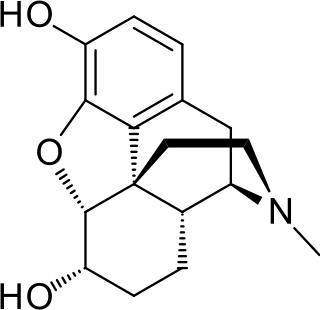
Dihydromorphine is a semi-synthetic opioid structurally related to and derived from morphine. The 7,8-double bond in morphine is reduced to a single bond to get dihydromorphine. Dihydromorphine is a moderately strong analgesic and is used clinically in the treatment of pain and also is an active metabolite of the analgesic opioid drug dihydrocodeine. Dihydromorphine occurs in trace quantities in assays of opium on occasion, as does dihydrocodeine, dihydrothebaine, tetrahydrothebaine, etc. The process for manufacturing dihydromorphine from morphine for pharmaceutical use was developed in Germany in the late 19th century, with the synthesis being published in 1900 and the drug introduced clinically as Paramorfan shortly thereafter. A high-yield synthesis from tetrahydrothebaine was later developed.

The Controlled Substances Penalties Amendments Act of 1984, 98 Stat. 2068, generally enhanced the penalties for violations of the Comprehensive Drug Abuse Prevention and Control Act of 1970. The 1984 legislation removed an ambiguity in the then-existing law by providing that a State drug felony conviction would trigger the provisions enhancing penalties for recidivists; it went further by providing that a Foreign drug felony conviction would have the same effect. Finally, the 1984 legislation doubled the penalties for distribution of controlled substances where the offense is committed on or within 1,000 feet of school property.

Levomethorphan (LVM) (INN, BAN) is an opioid analgesic of the morphinan family that has never been marketed. It is the L-stereoisomer of racemethorphan (methorphan). The effects of the two isomers of racemethorphan are quite different, with dextromethorphan (DXM) being an antitussive at low doses and a dissociative hallucinogen at much higher doses. Levomethorphan is about five times stronger than morphine.

Thebacon, or dihydrocodeinone enol acetate, is a semisynthetic opioid that is similar to hydrocodone and is most commonly synthesised from thebaine. Thebacon was invented in Germany in 1924, four years after the first synthesis of hydrocodone. Thebacon is a derivative of acetyldihydrocodeine, where only the 6–7 double bond is saturated. Thebacon is marketed as its hydrochloride salt under the trade name Acedicon, and as its bitartrate under Diacodin and other trade names. The hydrochloride salt has a free base conversion ratio of 0.846. Other salts used in research and other settings include thebacon's phosphate, hydrobromide, citrate, hydroiodide, and sulfate.

Nicocodeine is an opioid analgesic and cough suppressant, an ester of codeine closely related to dihydrocodeine and the codeine analogue of nicomorphine. It is not commonly used in most countries, but has activity similar to other opiates. Nicocodeine and nicomorphine were synthesized in 1904, and introduced in 1957 by Lannacher Heilmittel of Austria. Nicocodeine is metabolised in the liver by demethylation to produce nicomorphine, also known as 6-nicotinoylmorphine, and subsequently further metabolised to morphine. Side effects are similar to those of other opiates and include itching, nausea and respiratory depression. Related opioid analogues such as nicomorphine and nicodicodeine were first synthesized. The definitive synthesis, which involves treating anhydrous codeine base with nicotinic anhydride at 130 °C, was published by Pongratz and Zirm in Monatshefte für Chemie in 1957, simultaneously with the two analogues in an article about amides and esters of various organic acids.

Benzylmorphine (Peronine) is a semi-synthetic opioid narcotic introduced to the international market in 1896 and that of the United States very shortly thereafter. It is much like codeine, containing a benzyl group attached to the morphine molecule just as the methyl group creates codeine and the ethyl group creates ethylmorphine or dionine. It is about 90% as strong as codeine by weight.
A drug policy is the policy regarding the control and regulation of psychoactive substances, particularly those that are addictive or cause physical and mental dependence. While drug policies are generally implemented by governments, entities at all levels may have specific policies related to drugs.
The Opium Law is the section of the Dutch law which covers nearly all psychotropic drugs.
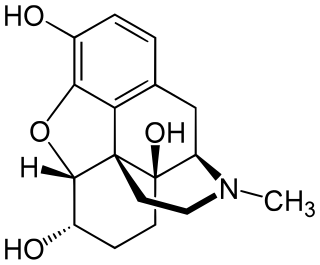
Hydromorphinol, is an opiate analogue that is a derivative of morphine, where the 14-position has been hydroxylated and the 7,8- double bond saturated. It has similar effects to morphine such as sedation, analgesia and respiratory depression, but is twice as potent as morphine and has a steeper dose-response curve and longer half-life. It is used in medicine as the bitartrate salt and hydrochloride

The Convention on Psychotropic Substances of 1971 is a United Nations treaty designed to control psychoactive drugs such as amphetamine-type stimulants, barbiturates, benzodiazepines, and psychedelics signed in Vienna, Austria on 21 February 1971. The Single Convention on Narcotic Drugs of 1961 did not ban the many newly discovered psychotropics, since its scope was limited to drugs with cannabis, coca and opium-like effects.
Legal cultivation of opium for medicinal purposes is carried out in India, only in selected areas, under free licensing conditions. India is the world's largest manufacturer of legal opium for the pharmaceutical industry according to the CIA World Factbook. India is one among 12 countries in world where legal cultivation for medical use is permissible within the ambit of United Nations, Single Convention on Narcotic Drugs 1961. In India legal cultivation is done primarily in Madhya Pradesh, Rajasthan and Uttar Pradesh. Despite producing poppy for opium production India depends heavily on imports to meet need of Poppy seed for edible purposes and domestic Codeine demand for medical purposes . Opium is heavily imported from its top producing nations like Afghanistan. There is also an account of Opium black marketing in India.
The major drug laws of India are the Narcotic Drugs and Psychotropic Substances Act (1985) and the Prevention of Illicit Trafficking in Narcotic Drugs and Psychotropic Substances Act (1988).
"International drug convention" could mean any of several international conventions:
References
- ↑ Wright, Quincy (1934). "The Narcotics Convention of 1931". American Journal of International Law. 28 (3): 475–486. doi:10.2307/2190375. ISSN 0002-9300. JSTOR 2190375.
- ↑ Pub.Res. 71–130, 46 Stat. 1516, enacted 3 March 1931
- ↑ Pub.Res. 71–136, 46 Stat. 1628, enacted 4 March 1931
- ↑ Krishnamoorthy, E. S. (1962). "Comparative analysis of the Permanent Central Opium Board and Drug Supervisory Body and their functions, on the one hand, and of the future International Control Board and its functions, on the other" Bulletin on Narcotics". United Nations Office on Drugs and Crime . Archived from the original on 28 February 2022. Retrieved 28 February 2022.
- ↑ Office international d'Hygiène publique (1933). Vingt-cinq ans d'activité de l'Office international d'Hygiène publique (1909-1933) (PDF) (in French). Paris: Office international d'hygiène publique.
{{cite book}}: CS1 maint: date and year (link) - ↑ "Cannabis amnesia – Indian hemp parley at the Office International d'Hygiène Publique in 1935 [preprint]". www.authorea.com. doi:10.22541/au.165237542.24089054/v1 (inactive 31 January 2024). Retrieved 3 December 2022.
{{cite web}}: CS1 maint: DOI inactive as of January 2024 (link)